
Prognostic impact of neutrophil-lymphocyte ratio in head and neck cancer: a decade of evidence
Introduction
In the prognostic landscape of oncology, the neutrophil-to-lymphocyte ratio (NLR) has emerged as a pivotal marker, particularly in solid tumours such as those affecting the liver and ovary (1). Its utility across various malignancies underscores a growing recognition of inflammatory markers in predicting patient outcomes and informing treatment pathways. Since every cancer patient undergoes a routine full blood count before treatment initiation, NLR provides an accessible measure of systemic inflammation—a factor increasingly linked to prognosis in oncology (2,3).
In head and neck squamous cell carcinoma (HNSCC), despite advancements in treatment modalities, accurate prognostication and personalized therapeutic strategies remain challenging. Elevated NLR, generally defined as greater than 3 in the context of malignancies, has been associated with systemic inflammation and a poorer prognosis. Normal NLR values typically range from 1 to 3, offering a comparative baseline for interpreting elevated levels in cancer patients. However, many studies investigating NLR in HNSCC are limited by small sample sizes, insufficient stratification by human papillomavirus (HPV) status, or lack of differentiation between treatment modalities. These gaps have hindered the establishment of NLR as a robust prognostic marker in routine clinical practice (4,5).
Recent research has highlighted the prognostic relevance of NLR in HNSCC across diverse treatment modalities and tumour sites (6,7). For instance, Yang et al. (6) demonstrated in a systematic review that elevated NLR correlates with adverse outcomes, advocating its inclusion in prognostic assessments. Additionally, studies exploring specific patient subgroups, such as those undergoing palliative care or chemoradiotherapy, underscore NLR’s broad applicability within HNSCC treatment paradigms (8).
This study aims to address these knowledge gaps by examining the prognostic significance of NLR in a large, population-based Western Australian cohort of HNSCC patients treated with diverse modalities. By evaluating the relationship between NLR and clinical outcomes, this research seeks to validate NLR as a non-invasive biomarker and improve its integration into prognostic frameworks for precision medicine in HNSCC.
Methods
Study design and population
This retrospective cohort study was conducted at a single centre in Western Australia, including patients diagnosed with HNSCC from January 1, 2013, to December 31, 2023. This study is reported according to the STROBE reporting guidelines (available at https://www.theajo.com/article/view/10.21037/ajo-24-52/rc). The study included patients treated with surgery, radiation therapy, or chemoradiotherapy. Exclusion criteria were concurrent malignancies, prior treatment before baseline blood work, haematological or endocrine malignancies, and infectious or inflammatory conditions that could influence NLR. Recurrence was identified clinically, radiologically, or via biopsy, encompassing local, regional, and distant recurrences. Follow-up duration ranged from 5 to 10 years (median: 7.5 years).
Data collection
Data were retrospectively collected from electronic medical records, capturing demographic details, smoking and alcohol history, primary tumour site, tumor, node, metastasis (TNM) staging, histopathological grading, HPV status, treatment modalities, and follow-up outcomes. NLR was calculated using absolute neutrophil and lymphocyte counts from blood samples collected within two weeks prior to treatment initiation.
Outcome measures
The primary outcome was overall survival (OS), defined as the time from diagnosis to death from any cause. Secondary outcomes included disease-free survival (DFS), measured as the interval from diagnosis to recurrence or death, and recurrence rates. Survival data were cross-referenced with the Western Australian Department of Health and the national death index to ensure accuracy.
Statistical analysis
NLR was analyzed as both a continuous variable and dichotomized using an optimal cutoff determined by receiver operating characteristic (ROC) curve analysis, employing Youden’s Index. Kaplan-Meier curves were used to estimate survival functions, and the log-rank test assessed differences between survival curves. Cox proportional hazards models evaluated the association between NLR and survival outcomes, adjusting for confounders such as age, sex, TNM stage, HPV status, and treatment modality. All statistical analyses were performed using SPSS Statistics Version 26 (IBM Corp., USA), with significance defined as P<0.05.
Ethical considerations
This study adhered to the Declaration of Helsinki (as revised in 2013) and received approval from the Western Australian Health Department Ethics Committee (No. RGS-06144). Due to its retrospective nature, patient consent was waived. All patient data were anonymized to ensure confidentiality.
Results
Demographic and clinical characteristics (Table 1)
Table 1
| Characteristic | Value |
|---|---|
| Age (years) | |
| Mean ± SD | 67.92±12.55 |
| Median | 69 |
| Gender | |
| Male | 743 (80.2%) |
| Female | 184 (19.8%) |
| Smoking | |
| Non-smokers | 316 (34.1%) |
| Smokers | 611 (65.9%) |
| Alcohol use | |
| Non-drinkers | 577 (62.2%) |
| Heavy drinkers | 350 (37.8%) |
| Ethnicity | |
| Aboriginal/Torres Strait Islanders | 21 (2.3%) |
| Non-Aboriginal | 906 (97.7%) |
SD, standard deviation.
This ten-year study included 927 patients diagnosed with HNSCC. The mean age was 67.92 (standard deviation =12.55) years, ranging from 21 to 93 years, with a median age of 69 years. The cohort was predominantly male, comprising 743 males (80.3%) and 184 females (19.7%).
Lifestyle factors varied across the cohort. Among patients, 316 (34.1%) were non-smokers, while 87 (9.4%) reported a smoking intensity of 20 pack-years. Regarding alcohol use, 577 patients (62.2%) reported no alcohol consumption, whereas 350 (37.8%) were classified as heavy drinkers.
Of the cohort, 21 patients (2.3%) identified as Aboriginal or Torres Strait Islanders, a figure lower than the general Australian representation of 3.8%. The remaining 906 patients (97.7%) were non-Indigenous Australians.
Cancer characteristics revealed the oropharynx as the most common subsite, accounting for 258 cases (27.8%). Of these, 200 patients (77.5%) were positive for HPV.
NLR and clinical outcomes
NLR was analysed in relation to various clinical outcomes. Elevated NLR, defined as >3, was significantly associated with recurrence, progression, or metastasis, as well as increased complications. A Pearson correlation coefficient of 0.52 [P<0.001, 95% confidence interval (CI): 0.47, 0.56] demonstrated a moderate positive relationship between NLR and adverse events. Similarly, complications correlated with NLR (r=0.35, P<0.001, 95% CI: 19.6, 83.4).
When stratified by NLR values, 370 of 500 patients with high NLR experienced recurrence, compared to 3 of 427 patients with low NLR [odds ratio (OR) =680.5, P<0.001, 95% CI: 213.5, 2,160.6]. For complications, 187 of 500 high NLR patients experienced adverse events, compared to 8 of 427 low NLR patients (OR =40.5, P<0.001, 95% CI: 19.6, 83.4).
Figures 1,2 depict these findings, highlighting the higher frequency of adverse outcomes in the high NLR group. “Recurrence/progression/metastasis by NLR group” and “Complications by NLR group” emphasize the clinical implications of NLR.


Prognostic value across treatment modalities (Figure 3, Table 2)
Table 2
| Treatment modality | Outcome | Odds ratio | P value | 95% confidence interval |
|---|---|---|---|---|
| Chemoradiotherapy | Complications | 18.9 | <0.001 | (7.2, 49.6) |
| Recurrence/progression/metastasis | 1,006.5 | <0.001 | (262.0, 3,866.8) | |
| Surgery + chemoradiotherapy | Complications | 2.95E+07 | <0.001 | (1.4E+07, 34E+07) |
| Recurrence/progression/metastasis | 8.23E+07 | <0.001 | (2E+07, 112E+07) | |
| Surgery + radiotherapy | Complications | 25.31 | <0.001 | (9.5, 66.9) |
| Recurrence/progression/metastasis | 55.5 | <0.001 | (23.7, 130) | |
| Surgery alone | Complications | 4.07E+12 | <0.001 | (1.5E+12, 11E+12) |
NLR, neutrophil-to-lymphocyte ratio.
NLR’s prognostic significance varied across treatment modalities. For patients undergoing chemoradiotherapy, high NLR was associated with increased complications (OR =18.9, P<0.001, 95% CI: 7.2, 49.6) and recurrence (OR =1,006.5, P<0.001, 95% CI: 262.0, 3,866.8). In patients receiving surgery combined with radiotherapy, elevated NLR was linked to complications (OR =25.3, P<0.001, 95% CI: 9.5, 66.9).
In surgery combined with chemoradiotherapy, results showed wide confidence intervals for complications (e.g., OR =29.5×106, 95% CI: 1.4E+07, 34E+07), reflecting variability and limitations in sample size. Results for radiotherapy or surgery alone demonstrated similar inconsistencies. These limitations underscore the complexity of NLR as a biomarker.
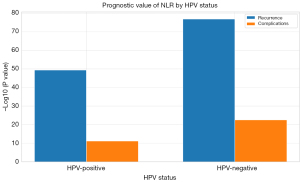
Impact of HPV status on prognostic value of NLR (Table 3)
Table 3
| HPV status & outcome | Odds ratio | P value | 95% CI lower | 95% CI upper |
|---|---|---|---|---|
| HPV-positive recurrence | 0.03 | <0.001 | 0.02 | 0.03 |
| HPV-positive complications | 0.08 | <0.001 | 0.06 | 0.09 |
| HPV-negative recurrence | 0.03 | <0.001 | 0.02 | 0.03 |
| HPV-negative complications | 0.28 | <0.001 | 0.23 | 0.34 |
CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; HPV, human papillomavirus.
For oropharyngeal HNSCC, NLR was evaluated according to HPV status. Among HPV-positive patients, high NLR correlated with recurrence (OR =0.03, P<0.001) and complications (OR =0.08, P<0.001). Similarly, in HPV-negative patients, high NLR was associated with recurrence (OR =0.03, P<0.001) and complications (OR =0.28, P<0.001).
Figure 4 highlights these associations, employing negative logarithmic transformations of P values to emphasize statistical significance.
Survival analysis based on NLR
Kaplan-Meier survival analysis (Figure 5) revealed significant differences in survival outcomes between high and low NLR groups. Patients with high NLR had a median survival of 5.46 years, while the median survival for low NLR patients could not be determined due to improved outcomes. The hazard ratio was 9.02 (P<0.001, 95% CI: 0.08, 0.15), underscoring the significant impact of elevated NLR on survival outcomes.

Discussion
This ten-year retrospective study highlights the NLR as a significant prognostic marker in HNSCC. This study represents comprehensive evaluations of NLR in HNSCC, featuring a large sample size and analysis across diverse treatment modalities and HPV status (4,9-11).
This study looked at 927 cases and found that most patients were on average 67.92 years old and mostly male, matching international data in HNSCC. The demographic and clinical characteristics of the study cohort align with global patterns, with a median age of 69 years and a male predominance of 80.3% (12). The underrepresentation of Aboriginal and Torres Strait Islander patients (2.3%) compared to the Australian population (3.8%) may reflect barriers to healthcare access or differences in disease prevalence, emphasizing the importance of equitable health interventions. Lifestyle risk factors, such as smoking and heavy alcohol consumption, were prevalent in this cohort, with 65% identified as smokers and 37.8% as heavy drinkers. These behaviours are known to promote systemic inflammation, potentially exacerbating elevated NLR levels and contributing to poorer outcomes. Addressing these modifiable risk factors through targeted behavioural interventions could significantly improve prevention and treatment outcomes.
Elevated NLR was strongly associated with increased recurrence, complications, and reduced survival. This finding aligns with prior studies that have demonstrated NLR’s utility as an inflammation-based prognostic biomarker in various malignancies, including HNSCC and other cancer types (5,13-15). Specifically, elevated NLR correlated with poorer outcomes in chemoradiotherapy and surgery combined with radiotherapy, consistent with the inflammatory burden’s impact on treatment efficacy. However, variability in the strength of associations across treatment modalities highlights the need for careful interpretation, particularly in subgroups with wide CIs or small sample sizes.
Our results align with studies from other types of cancer, showing that NLR is widely useful as a sign of inflammation in the body and can be a general predictor of how patients might do in the future (16,17). This assertion is particularly substantiated by our analysis among HPV in oropharyngeal cancer-patients, where elevated NLR significantly forecasts both higher recurrence and complication rates regardless of the HPV status (ORs of 0.03 for HPV positive and 0.28 for HPV negative). Such consisting predictive outcome shows that beyond the known impact of HPV on HNSCC prognosis, where HPV-positive patients often fare better, NLR stands out as an independent guide for clinical decisions across the board (18,19). Thus, NLR goes beyond traditional factors like HPV status, offering deep insights into cancer’s inflammatory processes and playing a crucial role in tailoring patient care strategies.
Notably, Kaplan-Meier survival analysis revealed that patients with elevated NLR exhibited significantly shorter survival durations (median: 5.46 years) compared to patients with low NLR, whose survival outcomes were markedly better. This highlights the importance of early identification of high-risk individuals to optimize management strategies and improve OS. The extreme ORs observed for recurrence (e.g., OR =680.51) and complications warrant consideration of potential statistical limitations, such as collinearity or small subgroup sizes, which may have amplified these estimates. Wide CIs in some analyses, particularly in patients receiving combined treatments, further underscore the need for larger, prospective studies to validate these findings and refine their clinical applicability.
This study also emphasizes the independent prognostic role of NLR in HPV-positive and HPV-negative oropharyngeal cancers. Elevated NLR was consistently associated with increased recurrence and complications in both HPV-positive and HPV-negative patients, demonstrating its utility across these biologically distinct subgroups. While HPV-positive patients typically exhibit better survival outcomes, these findings highlight NLR’s ability to provide additional prognostic insights beyond HPV status.
Despite its strengths, including a large sample size and comprehensive analysis, the study has limitations. Its retrospective design relies on historical medical records, which may vary in accuracy and completeness over the study period. Additionally, the use of single-centre data may limit generalizability to broader populations. Future research should prioritize prospective validation of these findings and explore the biological mechanisms linking systemic inflammation, as indicated by NLR, to cancer progression in HNSCC.
Looking ahead, the integration of NLR into clinical prognostic models is recommended to guide risk-adapted therapeutic interventions. By leveraging NLR as a non-invasive biomarker, clinicians can tailor treatment strategies to individual patients, ultimately improving outcomes and advancing personalized oncology care.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/10.21037/ajo-24-52/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-24-52/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-24-52/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-24-52/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Conducted in accordance with the Declaration of Helsinki (as revised in 2013), this study received approval from the Western Australian Health Department Ethics Committee (No. RGS-06144). Due to its retrospective nature, patient consent was waived, but confidentiality was meticulously preserved throughout the research process.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Templeton AJ, Rodríguez-Lescure Á, Ruíz A, et al. Prognostic role for the derived neutrophil-to-lymphocyte ratio in early breast cancer: a GEICAM/9906 substudy. Clin Transl Oncol 2018;20:1548-56. [Crossref] [PubMed]
- Song F, Cai H, Liao Y, et al. The systemic inflammation response index predicts the survival of patients with clinical T1-2N0 oral squamous cell carcinoma. Oral Dis 2022;28:600-10. [Crossref] [PubMed]
- Mikami T, Funayama A, Niimi K, et al. Prognostic value of preoperative systemic inflammatory response as a prognostic indicator in patients with early-stage oral squamous cell carcinoma. Medicine (Baltimore) 2022;101:e30855. [Crossref] [PubMed]
- Rachidi S, Wallace K, Wrangle JM, et al. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck 2016;38:E1068-74. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Yang L, Huang Y, Zhou L, et al. High pretreatment neutrophil-to-lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2019;41:1525-35. [Crossref] [PubMed]
- Fang Y, Yang Y, Chen M, et al. Elevated peripheral inflammatory markers are related with the recurrence and canceration of vocal fold leukoplakia. Eur Arch Otorhinolaryngol 2019;276:2857-64. [Crossref] [PubMed]
- Kim DY, Kim IS, Park SG, et al. Prognostic value of posttreatment neutrophil-lymphocyte ratio in head and neck squamous cell carcinoma treated by chemoradiotherapy. Auris Nasus Larynx 2017;44:199-204. [Crossref] [PubMed]
- Wong BY, Stafford ND, Green VL, et al. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Head Neck 2016;38:E1903-8. [Crossref] [PubMed]
- Bojaxhiu B, Templeton AJ, Elicin O, et al. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat Oncol 2018;13:216. [Crossref] [PubMed]
- Xun Y, Wang M, Sun H, et al. Prognostic Analysis of Preoperative Inflammatory Biomarkers in Patients With Laryngeal Squamous Cell Carcinoma. Ear Nose Throat J 2020;99:371-8. [Crossref] [PubMed]
- Pynnonen MA, Gillespie MB, Roman B, et al. Clinical Practice Guideline: Evaluation of the Neck Mass in Adults. Otolaryngol Head Neck Surg 2017;157:S1-S30. [Crossref] [PubMed]
- Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2. [Crossref] [PubMed]
- Zhou W, Peng C, Liu Z, et al. A novel clinical signature predicts the survival of elderly patients with oral squamous cell carcinoma. Eur Arch Otorhinolaryngol 2022;279:391-8. [Crossref] [PubMed]
- Salzano G, Perri F, Maglitto F, et al. Pre-Treatment Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Predictors of Occult Cervical Metastasis in Clinically Negative Neck Supraglottic and Glottic Cancer. J Pers Med 2021;11:1252. [Crossref] [PubMed]
- Yeh TJ, Wang HC, Cho SF, et al. The Prognosis Performance of a Neutrophil- and Lymphocyte-Associated Gene Mutation Score in a Head and Neck Cancer Cohort. Biomedicines 2023;11:3113. [Crossref] [PubMed]
- Chandrasekara S, Davis S, Thomson P, et al. High neutrophil-to-lymphocyte ratio predicts poor prognosis in patients with squamous cell carcinoma of the head and neck treated with definitive chemoradiotherapy. Asia Pac J Clin Oncol 2018;14:e442-7. [Crossref] [PubMed]
- Kędzierawski P, Huruk-Kuchinka A, Radowicz-Chil A, et al. Human papillomavirus infection predicts a better survival rate in patients with oropharyngeal cancer. Arch Med Sci 2021;17:1308-16. [Crossref] [PubMed]
- Pontillo A, Bricher P, Leal VN, et al. Role of inflammasome genetics in susceptibility to HPV infection and cervical cancer development. J Med Virol 2016;88:1646-51. [Crossref] [PubMed]
Cite this article as: Reyes-Chicuellar N, Kagan R, Friedland P. Prognostic impact of neutrophil-lymphocyte ratio in head and neck cancer: a decade of evidence. Aust J Otolaryngol 2025;8:13.



