
Pneumonia and unilateral vocal fold immobility: a single centre 6-year review
Introduction
One of the functions of the larynx is airway protection and prevention of aspiration facilitated partly by the mobility and closure of the vocal folds during swallowing (1). Unilateral vocal fold immobility (UVFI) is a clinical condition that may arise secondary to either mechanical or neurogenic factors (2). The commonly identified aetiologies include; iatrogenic (55–74%, neck/cardiac/thoracic surgery), idiopathic (5–29%) and thoracic or mediastinal malignancy (16–17%) (3-5).
Typically, UVFI is identified by symptoms including hoarseness and breathiness (6). However, in recent studies dysphagia has been recognized as another common feature in this patient population that further correlates to an increased risk of penetration and aspiration of food or fluids during swallowing (3,5). Dysphagia has been reported to occur in up to 56% to 69% of patients with UVFI with aspiration rates ranging from 23% to 53% (7). When UVFI is suspected, confirmation is made by a bedside flexible nasoendoscopy (FNE) by an otolaryngologist.
Initial management of UVFI generally involves the following three options: observation for spontaneous recovery, speech therapy and injection laryngoplasty (IL). IL typically utilises an absorbable substance (hyaluronic acid gels or calcium hydroxylapatite) injected into the paraglottic space, with the aim of medialising the affected vocal fold and decreasing glottal incompetence (8). IL to treat UVFI can be performed in the office or theatre setting for these patients (9). Depending on the severity of the injury causing the UVFI and underlying aetiology, spontaneous improvement of vocal fold function may occur within 6–12 months (10). A meta-analysis by Vila et al. however showed that patients who did not undergo early IL (<6 months from diagnosis) were subsequently four times more likely to go on to require a more invasive and permanent procedure (medialisation thyroplasty) compared to those who did undergo early IL (11).
In Australia, episodes of pneumonia of any cause requiring hospitalization have been reported to be over 123,000 episodes between 2017–2018 (12). Types of pneumonia include community and hospital acquired or aspiration pneumonia (13). In regards to aspiration pneumonia, this requires a consistent history of an aspiration event associated with clinical features of pneumonia and a chest X-ray that often displays lower zone consolidation (13). Our aim is to retrospectively review the occurrence of pneumonia in patients undergoing IL for UVFI. We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-50/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved through the low and negligible risk pathway by the Southern Adelaide Local Health Network HREC (LNR reference number: LNR/21/SAC/15) who deemed it met the requirements of the National Statement on Ethical Conduct in Human Research (2007, updated 2018) and the SALHN Research Governance policy. Because of the retrospective nature of the research, the requirement for informed consent was waived.
This is a retrospective cohort study of patients who underwent an IL for UVFI. Procedures were performed at Flinders Medical Centre, Adelaide between January 2015 and December 2020. Patient episodes were identified from the electronic Operating Room Management Information System (ORMIS) and hospital coding using the search terms: administration of agent into larynx or vocal cord, laryngoplasty, fold injection, thyroplasty, vocal fold palsy, cord injection, vocal palsy, and vc palsy. The Medicare Benefits Schedule (MBS) codes 41870 (injection of vocal cord by Teflon, fat, collagen or gelfoam) and 41876 (larynx, external operation on, or laryngofissure with or without cordectomy) were also used. Voice clinic records were manually searched for all patients who underwent an IL for UVFI in the clinic.
Data collected included: patient demographics, laterality and aetiology of the UVFI, number of admissions with pneumonia and the outcomes of Ear, Nose, and Throat (ENT) and speech pathology assessments. Of those who were seen by a speech pathologist, standard bedside assessment of oral and pharyngeal phases of swallowing function were undertaken. Oral phase assessment included factors such as: lip seal and bolus preparation (14). Pharyngeal phase assessments included timing of swallow, velopharyngeal insufficiency, laryngeal elevation, evidence of aspiration (14). Based on these assessments, a diagnosis of oral, pharyngeal or oropharyngeal dysphagia was documented.
Patients were followed up until death or the end of the study period (December 31, 2020). Our primary outcome was hospital admissions with pneumonia. Secondary outcomes included; post-IL pneumonia free survival and the prevalence of both subjective reports and objective assessments of dysphagia among patients with UVFI.
We performed descriptive statistical analysis to identify total number of patients or mean and range for relevant outcomes. For categorical data the Fisher’s Exact and Chi square tests were used for 2 × 2 and n × m contingency tables respectively. Statistical significance was defined as P<0.05. Time to event analysis was estimated with the Kaplan-Meier method. All statistical analyses were performed using SPSS version 27 (IBM Corp., Armonk, NY, USA).
Results
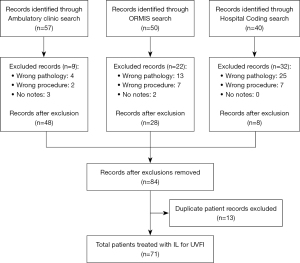
A total of 147 patients were initially identified using the above search criteria. Reasons for exclusion included: incorrect procedure coded (n=16, e.g., external framework thyroplasty or injection of botulinum toxin type A), injection for different pathology (n=42, e.g., spasmodic dysphonia, presbyphonia, stenosis of larynx, nodules of vocal cords) or no available notes (n=5). Once records from the three databases were combined, 13 duplicate patient records were removed. Our final sample included 71 patients who had undergone an IL for UVFI, see Figure 1. IL was performed using a hyaluronic acid filler (Restylane or Restylane Lyft; Galderma Laboratories, Fort Worth, Texas, USA). Procedures were performed either in the Flinders Medical Centre Outpatient Ambulatory Clinic (n=40) with local anaesthesia or in the operating theatre (n=31) under sedation or general anaesthesia. The majority of patients had only undergone one IL by the end of the study period. Fifteen had undergone two ILs and four had undergone three ILs. The mean interval between subsequent ILs was 12 months (range, 2 to 37 months).
The mean age of the study patients was 63 years (range, 15–91 years) with a slight predominance of female (n=38) patients compared to male (n=33). UVFI was left sided in 48 patients and right sided in 23 patients. The common aetiologies were iatrogenic (n=24), malignancy (n=15), stroke (n=11) and idiopathic (n=10). Of the iatrogenic causes, most were post-thyroidectomy (n=8) followed by cardiothoracic surgery (n=5) and anterior cervical discectomy and fusion (n=4). Patient demographics are shown in Table 1.
Table 1
| Demographics | Number [%] |
|---|---|
| Gender | |
| Female | 38 [54] |
| Male | 33 [46] |
| Side of palsy | |
| Left | 48 [68] |
| Right | 23 [32] |
| Injection laryngoplasty performed in | |
| Theatre | 31 [44] |
| Clinic | 40 [56] |
| Aetiology of the unilateral vocal fold immobility | |
| Iatrogenic | 24 [33] |
| Thyroidectomy | 8 [11] |
| Cardiothoracic | 5 [7] |
| Other | 11 [15] |
| Malignancy | 15 [21] |
| Stroke | 11 [15] |
| Idiopathic | 10 [14] |
| Cardiac | 4 [6] |
| Trauma | 3 [4] |
| Congenital | 2 [3] |
| Thyroid goiter | 2 [3] |
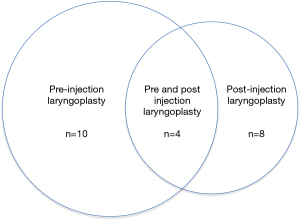
Overall, 22 of the 71 patients had admission(s) for pneumonia before and/or after undergoing IL (range, 1–6 admissions). The remaining 49 patients in our study did not have an admission with pneumonia either before or after IL. The 22 admissions with pneumonia occurred as follows: only before (n=10), both before and after (n=4) or only after (n=8) IL, see Figure 2.
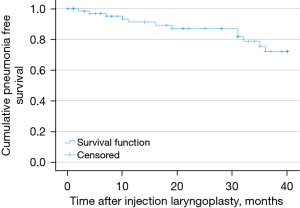
Twelve patients were found to have admissions with pneumonia after undergoing IL. For these patients the time between IL and admission with pneumonia ranged from 2 months to 3 years. Three-year pneumonia free survival following IL was therefore 83%, see Figure 3. Only two of these 12 patients had an admission with pneumonia within 7 months after undergoing IL. The case history of these two patients will be presented next.
The first of these two patients was a 60-year-old female with liver, ribs and skull base metastases secondary to uterine cancer. She developed UVFI secondary to her skull base metastases and was treated with IL. She was subsequently admitted with pneumonia 4 months after IL. During this admission, FNE by the otolaryngology team noted a glottic gap on phonation.
The second of these patients was a 60-year-old female with bone, spine, lung and brain metastases secondary to breast cancer. She was admitted to hospital with pneumonia 2 months after IL. During her admission the patient denied any swallowing difficulties with an improved voice since IL. Speech pathology assessment did not identify overt signs of aspiration and recommended a full ward diet. There was no documentation identified regarding otolaryngology input or FNE during this admission.
The relationship between patient specific factors and their rates of admission with pneumonia post IL has been summarized in Table 2. We found that patients with Iatrogenic UVFI were significantly less likely than non-Iatrogenic UVFI to be admitted with pneumonia post IL [4% (n=1) versus 23% (n=11) respectively, odds ratio (OR) 7.03; 95% confidence interval (CI): 0.85–58.14, P=0.04]. Of the non-iatrogenic causes, patients were also significantly more likely to be admitted with pneumonia post IL if their UVFI was secondary to a structural cardiac cause [n=2 (50%), OR 23.00; 95% CI: 1.40–379.90; P=0.05] or a stroke [n=4 (36%); OR 13.14; 95% CI: 1.26–137.67; P=0.03]. We also found a higher proportion of patients who underwent IL in theatre compared to in clinic were admitted with pneumonia following their procedure [n=9 (29%) versus n=3 (8%) respectively; OR 5.05; 95% CI: 1.23–20.65; P=0.02].
Table 2
| Patient assessments/factors | Patients admitted for pneumonia post IL, n [%] | Odds ratio (95% CI) | P value |
|---|---|---|---|
| Cause | |||
| Iatrogenic | 1 [4] | ||
| Malignancy | 3 [20] | 5.75 (0.54–61.41) | 0.11 |
| Cardiac | 2 [50] | 23.00 (1.40–379.90) | 0.05 |
| Congenital | 0 | ||
| Idiopathic | 2 [20] | 5.75 (0.46–72.30) | 0.20 |
| Stroke | 4 [36] | 13.14 (1.26–137.67) | 0.03 |
| Thyroid | 0 | ||
| Trauma | 0 | ||
| Non-iatrogenic | 11 [23] | 7.03 (0.85–58.14) | 0.04 |
| Injection location | |||
| Clinic | 3 [8] | ||
| Theatre | 9 [29] | 5.05 (1.23–20.65) | 0.02 |
| Speech pathology assessments | |||
| Normal swallow | 2 [29] | ||
| Pharyngeal dysphagia | 2 [15] | 2.20 (0.24–20.40) | 0.44 |
| Oropharyngeal dysphagia | 5 [26] | 1.12 (0.16–7.73) | 0.64 |
In regards to objective swallow assessment, there were 21 patients who had no documented assessment by a speech pathologist during their hospital admissions, two of which had reported dysphagia. Of the 50 patients that were reviewed by a speech pathologist, 11 reported no difficulty swallowing, 7 had a normal swallow assessment, 19 were assessed as oropharyngeal dysphagia and 13 assessed as pharyngeal dysphagia. Overall, 34 of the 71 (48%) patients reported dysphagia and/or an objective assessment of dysphagia was documented in the medical record by a speech pathologist. When we compared the 50 patients who were assessed by a speech pathologist, we found those with a documented normal swallow had the highest rate of admission(s) for pneumonia post IL, n=2 (29%). There were no significant differences in rates of admission(s) with pneumonia post IL when we compared those with a normal swallow assessment versus those with documented; pharyngeal dysphagia [n=2 (15%); OR 2.20; 95% CI: 0.24–20.40; P=0.44] or oropharyngeal dysphagia [n=5 (26%); OR 1.12; 95% CI: 0.16–7.73; P=0.64].
Discussion
Patients with UVFI are usually symptomatic due to dysphonia but are now recognized increasingly to have some form of coexisting dysphagia and an increased risk of pneumonia (3,15-17). Our study identified that 22 of 71 patients (31%) with UVFI had admissions with pneumonia. This finding supports a nationwide retrospective study from Taiwan comparing 417 patients with newly diagnosed UVFI to a separate group without UVFI (17). In this study Tsai et al. recorded a higher 8-year cumulative incidence rate of pneumonia in patients with UVFI compared to those without, 19% and 13% respectively (17).
We found that 14 of 71 patients with UVFI had previous admission(s) with pneumonia prior to undergoing IL. Of these, only four had further hospital admissions for pneumonia after treatment with IL. This suggests treatment of UVFI with IL potentially reduces the risk of subsequent admissions with pneumonia. A retrospective review by Barnes et al. in 2020 supports this finding where patients who underwent IL for UVFI were less likely to develop pneumonia compared to those with UVFI who were not treated [hazard ratio (HR) 0.33, 95% CI: 0.11–0.98; P=0.045] (18). This study by Barnes et al. retrospectively analysed pneumonia rates in patients with iatrogenic UVFI secondary to cardiothoracic surgery (18). Similarly, in a subgroup analysis of patients in our study, we found that patients with iatrogenic UVFI were significantly less likely to be admitted with pneumonia post IL than other aetiologies. Potentially our findings in the iatrogenic UVFI population may represent a benefit with early IL in patients with iatrogenic UVFI in reducing the risk of hospital admission with aspiration pneumonia. When comparing post IL pneumonia rates based on procedure location, we found better outcomes in those who had their procedure in clinic versus in theatre. This finding may be a secondary to the ability to make a better intra-procedural assessment of glottic closure on phonation in a patient who is self-ventilating and not sedated.
We are cautious however not to overstate the perceived benefit for reducing hospital admissions with recurrent pneumonia in regards to treatment of UVFI with an IL. This is because we also identified a cohort of patients who were admitted with pneumonia several months after their procedure. Our results demonstrate most of these admissions were more than 7 months following IL. Only two admissions occurred within 4 months of IL. The patients that did have admissions with pneumonia more than 7 months after their IL are likely explained by the absorbable and temporary nature of the injected hyaluronic acid filler. Restylane injectable filler has been reported to have effects that generally last for up to 4-to-6 months (19). Once absorbed, if the vocal fold returns to a state where there is incomplete glottic closure the patient is predisposed to aspiration pneumonia.
The patient that was admitted 4 months following their IL was identified to have a new glottic gap that had developed at some stage between their procedure and time of review. This finding may be a reflection of the temporary nature of the injected material absorbing over time. In regards to the second patient who developed pneumonia post IL, details of their admission suggest the aetiology of pneumonia may not have been secondary to aspiration. They presented with no dysphagia and an improved voice quality following IL. The speech pathologist assessed their swallow as safe during their admission however an FNE or fiberoptic endoscopic evaluation of swallowing (FEES) was not performed to assess for the presence or absence of aspiration.
The demographic factors from our cohort of patients reflect what has been previously documented in the literature. Our results are consistent with previous findings of UVFI being more common on the left side with iatrogenic and thoracic or mediastinal malignancy being the most common aetiologies (3-5). When assessing the prevalence of dysphagia among patients with UVFI we found that 48% of our study patients had dysphagia. The accuracy of this result is limited however by the various ways in which we define dysphagia from subjective patient reported measures to more invasive objective assessments. A recent study by Schiedermayer et al. reported on the prevalence of dysphagia in a population of 415 patients with UVFI comparing multiple different definitions of dysphagia (16). This study found varying rates of dysphagia within the same cohort based on the way in which dysphagia was reported. For example, dysphagia rates ranged from 48% based on patient reported outcome measures to 19% in those where the diagnosis of dysphagia was based on objective findings of abnormal swallow function on instrumental assessments [FEES or modified barium swallow (MBS)]. Overall, 62% were documented with dysphagia based on at least one of their definitions. In another study by Anis et al. of 21 patients with UVFI, 90% had dysphagia as assessed with a patient reported outcome measure [Eating Assessment Tool-10 (EAT-10) questionnaire] (15).
The presence of dysphagia and impaired glottis closure in patients with UVFI predisposes these patients to aspiration pneumonia. We were able to identify 20% of our UVFI cohort who had admissions for pneumonia prior to undergoing IL. A limitation of this retrospective study however is that we were unable to differentiate the type of pneumonia due to the lack of documentation in patients’ case notes. Therefore, we may be overestimating the true number of admissions for aspiration pneumonia in our cohort.
Further limitations with the retrospective nature of this study are the reliance on documentation in medical records for data collection. Episodes of pneumonia where patients were treated in the primary care setting would have been missed in our study potentially underestimating its prevalence. Dysphagia in this cohort of patients may also have been secondary to other causes unrelated to their UVFI. Another limitation of our retrospective study was that we were unable to identify a cohort of patients admitted with aspiration pneumonia and normal vocal fold function or alternatively a group of patients with UVFI that did not undergo IL to use as a comparator group.
Conclusions
UVFI is an important health problem that can be diagnosed effectively by an otolaryngologist at the patients’ bedside. UVFI is also a condition whereby safe treatment in the form of IL is effective. The current gap in knowledge is our understanding of swallowing mechanics in relation to UVFI and response to IL. Recognition of recurrent episodes of pneumonia in the setting of a change in voice or swallow function should prompt early referral to speech pathology and otolaryngology to assess for UVFI as intervention with an IL could reduce the risk of hospital admissions for recurrent pneumonia. In addition, there may be a role for these patients to be followed up more closely around the time of expected duration of the augmentation gel as resorption around the 4-to-6-month mark may then predispose them to further aspiration.
Acknowledgments
We would like to thank the Flinders Medical Centre electronic Operating Room Management Information System (ORMIS) and hospital coding teams for assistance collating data. This research has been presented at the Australian Society of Otolaryngology, Head and Neck Surgery 72nd Annual Scientific Meeting.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-50/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-50/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-50/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-50/coif). T.A. serves as an unpaid editorial board member of Australian Journal of Otolaryngology from January 2019 to December 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved through the low and negligible risk pathway by the Southern Adelaide Local Health Network HREC (LNR reference number: LNR/21/SAC/15) who deemed it met the requirements of the National Statement on Ethical Conduct in Human Research (2007, updated 2018) and the SALHN Research Governance policy. Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jafari S, Prince RA, Kim DY, et al. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol 2003;550:287-304. [Crossref] [PubMed]
- Zhou D, Jafri M, Husain I. Identifying the Prevalence of Dysphagia among Patients Diagnosed with Unilateral Vocal Fold Immobility. Otolaryngol Head Neck Surg 2019;160:955-64. [Crossref] [PubMed]
- Bhattacharyya N, Kotz T, Shapiro J. Dysphagia and aspiration with unilateral vocal cord immobility: incidence, characterization, and response to surgical treatment. Ann Otol Rhinol Laryngol 2002;111:672-9. [Crossref] [PubMed]
- Cates DJ, Venkatesan NN, Strong B, et al. Effect of Vocal Fold Medialization on Dysphagia in Patients with Unilateral Vocal Fold Immobility. Otolaryngol Head Neck Surg 2016;155:454-7. [Crossref] [PubMed]
- Nayak VK, Bhattacharyya N, Kotz T, et al. Patterns of swallowing failure following medialization in unilateral vocal fold immobility. Laryngoscope 2002;112:1840-4. [Crossref] [PubMed]
- Tateya I, Hirano S, Kishimoto Y, et al. Impacts and limitations of medialization thyroplasty on swallowing function of patients with unilateral vocal fold paralysis. Acta Otolaryngol Suppl 2010;84-7. [Crossref] [PubMed]
- Zuniga S, Ebersole B, Jamal N. Improved swallow outcomes after injection laryngoplasty in unilateral vocal fold immobility. Ear Nose Throat J 2018;97:250-6. [Crossref] [PubMed]
- Prendes BL, Yung KC, Likhterov I, et al. Long-term effects of injection laryngoplasty with a temporary agent on voice quality and vocal fold position. Laryngoscope 2012;122:2227-33. [Crossref] [PubMed]
- Chandran D, Woods C, Ullah S, et al. A comparative study of voice outcomes and complication rates in patients undergoing injection laryngoplasty performed under local versus general anaesthesia: an Adelaide voice specialist's experience. J Laryngol Otol 2017;131:S41-6. [Crossref] [PubMed]
- Granato F, Martelli F, Comini LV, et al. The surgical treatment of unilateral vocal cord paralysis (UVCP): qualitative review analysis and meta-analysis study. Eur Arch Otorhinolaryngol 2019;276:2649-59. [Crossref] [PubMed]
- Vila PM, Bhatt NK, Paniello RC. Early-injection laryngoplasty may lower risk of thyroplasty: A systematic review and meta-analysis. Laryngoscope 2018;128:935-40. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. Admitted patient care 2017-18 Australian Hospital Statistics. Australian Institute of Health and Welfare, Australian Institute of Health and Welfare. Accessed 14 January 2024. Available online: https://www.aihw.gov.au/getmedia/df0abd15-5dd8-4a56-94fa-c9ab68690e18/aihw-hse-225.pdf
- DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care 2015;30:40-8. [Crossref] [PubMed]
- Speech Pathology Learner Guide, Provide support in dysphagia management. Queensland Health, Queensland. 2017. Accessed 23 May 2022. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0025/650581/LG-dysphagia-mgmt.pdf
- Anis MM, Memon Z. Injection medialization laryngoplasty improves dysphagia in patients with unilateral vocal fold immobility. World J Otorhinolaryngol Head Neck Surg 2018;4:126-9. [Crossref] [PubMed]
- Schiedermayer B, Kendall KA, Stevens M, et al. Prevalence, incidence, and characteristics of dysphagia in those with unilateral vocal fold paralysis. Laryngoscope 2020;130:2397-404. [Crossref] [PubMed]
- Tsai MS, Yang YH, Liu CY, et al. Unilateral Vocal Fold Paralysis and Risk of Pneumonia: A Nationwide Population-Based Cohort Study. Otolaryngol Head Neck Surg 2018;158:896-903. [Crossref] [PubMed]
- Barnes JH, Orbelo DM, Armstrong MF, et al. Cardiothoracic Patients with Unilateral Vocal Fold Paralysis: Pneumonia Rates Following Injection Laryngoplasty. Ann Otol Rhinol Laryngol 2020;129:1129-34. [Crossref] [PubMed]
- King JM, Simpson CB. Modern injection augmentation for glottic insufficiency. Curr Opin Otolaryngol Head Neck Surg 2007;15:153-8. [Crossref] [PubMed]
Cite this article as: Lee TJ, Athanasiadis T, Ooi EH. Pneumonia and unilateral vocal fold immobility: a single centre 6-year review. Aust J Otolaryngol 2024;7:14.


