
Recommendations for audiometric follow up post paediatric bacterial meningitis based on an Australian experience: a retrospective cross-sectional study
Introduction
Prior to the wide availability and implementation of antibiotics, bacterial meningitis was thought to account for 20% of sensorineural hearing loss (SNHL) in children (1). Almost 50 years ago, Nadol published a review of 547 cases of paediatric meningitis of which 236 were bacterial in nature. Twenty-six (11.0%) children suffering bacterial meningitis died and of those whom survived, 28 (11.9%) were diagnosed with SNHL (2). Fortunately, in the last four decades our understanding of meningitis has progressed and death rates in developed countries are now below 5% (3,4).
A Cochrane review (5) of corticosteroid use in bacterial meningitis demonstrated that in high income countries, the incidence of SNHL was reduced in children treated with corticosteroid just prior to, or at commencement of antibiotic therapy. Regarding causative organism, corticosteroids were associated with a lower incidence of SNHL in meningitis associated with H. influenzae but not with S. pneumoniae. In Australia, the high vaccination rate against H. influenzae has significantly reduced the prevalence of associated meningitis, however S. pneumoniae meningitis remains of concern (6-8).
Despite high rates of childhood vaccination and improved hospital care, meningitis related hearing loss is still reported in approximately 5% of cases overall, with S. pneumoniae meningitis recording rates of hearing loss up to 32% (7). Hearing loss is theorised to occur due to direct extension of bacterial pathogens, or as a result of the associated inflammatory response. In the cochlea, this may lead to labyrinthitis ossificans which can occur in up to 80% of post S. pneumoniae meningitis induced SNHL. The timing of onset of labyrinthitis ossificans is poorly defined in humans but has been demonstrated to occur within 3 weeks of meningitis (9,10). In the setting of ossificans, the new bone formation within the cochlear can make cochlear implantation challenging or even impossible. As cochlear implantation is the gold standard of care for severe to profound SNHL, early implantation in this setting becomes critical. Currently, there is a lack of Australian data on audiology follow up and hearing outcomes of children diagnosed with bacterial meningitis by non-Otolaryngology specialists. Thus the aim of the study was to review the current referral and audiological follow up time of these children, including their hearing outcomes and offer a standardised referral guideline for audiological surveillance post bacterial meningitis. We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-45/rc).
Methods
A retrospective cross-sectional study of all children from birth to 18 years of age treated for bacterial meningitis at Queensland Children’s Hospital (QCH) between November 2014 and December 2021 (7 years). QCH, Brisbane, Queensland (opened in Nov 2014) is the major tertiary referral centre for the state which has a population of over 5 million.
Initially, a hospital database search was conducted to identify patients with a diagnosis of bacterial meningitis. Electronic medical records allowed this data to be obtained regardless of whether diagnosis and initial treatment occurred at QCH or at another Queensland Health facility within the state.
Once identified, medical records were reviewed and information collected on patient demographics, age at diagnosis, causative organism, length of hospital stay, audiological referral timeline and outcomes were recorded. Patients with a diagnosis of viral meningitis were excluded as were those with cerebrospinal fluid (CSF) features not in keeping with bacterial meningitis (white cell count, glucose and protein levels) despite the growth of an organism (deemed likely a contaminant). Descriptive analysis of data was undertaken.
PubMed database searches were conducted using the terms “meningitis”, “hearing loss”, “audiology”, “guidelines”, and “paediatric” in varying combinations of some and all terms. Articles not published in English language were excluded. A search of each Australian state health service website and of the New Zealand Health website was conducted to identify locally available referral guidelines.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was gained from Children’s Health Queensland Human Research Ethics Committee (project number 87811). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Results
A hospital database search identified 75 patients with a recorded diagnosis of bacterial meningitis. Review of medical records resulted in exclusion of 18 patients due to incorrect recording of the diagnosis, the most common reason for exclusion was admission due to viral meningitis. Of 57 patients identified, 38 were male (66.7%). Mean age at admission was 378 days (median 33 days, range, 2 days to 17 years) and 45 patients (78.9%) were fully immunised as per the Australian National Immunisation Program Schedule (8) (Table 1).
Table 1
| Variables | Age bracket | |||
|---|---|---|---|---|
| 0–30 days | 31 days–<1 year | 1–5 years | >5 years | |
| Total (n=57) | 27 | 25 | 1 | 4 |
| Male | 18 (66.7%) | 16 (64.0%) | 0 | 4 (100%) |
| Median age (range) | 15 days (2–30 days) | 76 days (32–205 days) | 2 years | 11 years (10–17 years) |
| Immunised* | 19 (70.4%) (unknown 1) | 21 (84.0%) (unknown 0) | 1 (100%) | 4 (100%) |
| Passed NBHS | 22 (81.5%) (unknown 2) | 21 (84.0%) (unknown 4) | 1 (100%) | 3 (75%) (unknown 1) |
| Streptococcus sp. | 1 | 2 (8.0%) | 0 | 2 |
| E. coli | 17 (63.0%) | 13 (52%) | 0 | 1 |
| Unknown organism | 5 | 5 | 2 | 0 |
| PICU admission | 8 | 2 | 1 | 3 |
| Mean duration of IV antibiotic therapy (range) | 21.7 days (5–44 days) | 21.1 days (10–42 days) | 7 days (no range) | 20.8 days (14–34 days) |
| Mean duration of hospital admission (range) | 37.4 days (4–386 days) | 13.5 days (3–52 days) | 9 days (no range) | 23.3 days (4–48 days) |
*, as per the Australian National Immunisation Program Schedule. NBHS, newborn hearing screen; PICU, paediatric intensive care unit; IV, intravenous.
E. coli and Streptococcus species were positive in 31 (54.4%) and 5 (8.8%) of these patients respectively. Twelve patients (21.1%) failed to identify an organism on CSF culture but were deemed likely to have bacterial meningitis based on clinical features and other laboratory findings. Other bacteria identified included Klebsiella, Serratia and Pasteurella. Mean duration of intravenous (IV) antibiotic therapy was 20.7 days (range, 5–42 days) with cefotaxime being the most common primary antibiotic used and mean duration of hospital stay was 25.2 days. Fourteen of 57 children (24.6%) were treated in paediatric intensive care.
In regards to audiological surveillance, 47 patients (82.5%) had a documented pass on their newborn hearing screen with 2 patients receiving a “refer” and another 8 patients (14.0%) unknown. Audiology referral was identified for 33 patients (58.6%) and an additional 13 (22.4%) attended audiology follow up but initial referral could not be located, thus 46 patients (80.7%) were reviewed by audiology post diagnosis. Eleven patients (19.3%) received no referral or audiological follow up post treatment. Forty-one of the 46 patients attended post treatment audiology review. Mean duration from date of hospital admission to audiological testing was 63.4 days (median 36.0 days, range, 8–304 days). Number of days between diagnosis and referral being made varied from 0 to 97 days (median 8.5 days) (Table 2). Of those with normal sensorineural hearing thresholds at first audiological review (n=38), 34 patients (89.5%) underwent repeat testing (mean time between tests, 165 days) and 31 patients (81.6%) had audiology performed on more than 2 occasions. There were no additional cases of SNHL identified on subsequent audiograms (Figure 1).
Table 2
| Variables | Number (% of total) | Median time (days) | Range (days) |
|---|---|---|---|
| Referred to audiology | 46 (80.7) | 8.5 | 0–97 |
| Attended audiology | 41 (71.9) | 36 | 8–304 |

Only 3 children (5.3%) were identified to have SNHL post meningitis, each cultured Streptococcus sp. from CSF (Table 3). Of these three patients, one proceeded to successful surgical bilateral cochlear implantation 93 days post presentation with meningitis. This patient experienced relative delay to audiological assessment at 59 days post admission but rapid escalation with Ear, Nose Throat (ENT) Specialist referral (seen 1 day after audiology) followed by radiological assessment to ensure absence of ossification and subsequent bilateral implantation. The remaining two patients suffering SNHL post meningitis did not proceed to cochlear implantation due to significant additional comorbidities acquired from the episode of meningitis.
Table 3
| Patient No. | Age | Organism | Immunised* | PICU duration (days) | Length of admission (days) | Hearing status post event | CI candidate | Implant outcome |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 2 days | S. pyogenes | N/A | 8 | 386 | Severe SNHL; ANSD | No | N/A |
| Patient 2 | 107 days | S. agalactiae | Y | 0 | 20 | Profound SNHL | Yes | Successful bilateral CI |
| Patient 3 | 150 days | S. pneumoniae | N | 0 | 13 | Normal hearing | No | N/A |
| Patient 4 | 10 years, 5 months | S. pneumoniae | Y | 0 | 48 | Normal hearing | No | N/A |
| Patient 5 | 17 years, 1 month | S. pneumoniae | Y | 1 | 4 | Profound SNHL | Yes | Declined CI |
*, as per the Australian National Immunisation Program Schedule. PICU, paediatric intensive care unit; CI, cochlear implantation; N/A, not applicable; SNHL, sensorineural hearing loss; ANSD, auditory neuropathy spectrum disorder; N, no; Y, yes.
Publicly available guidelines were identified from Royal Children’s Hospital, Melbourne (11), New Zealand Department of Health (12), and the United Kingdom (UK) (13).
Discussion
Over a 7-year period, QCH identified and treated 57 children with bacterial meningitis. Similar to current literature, this was most prevalent in those less than 1 year of age, with the median age in this subgroup being 30 days (7). Interestingly, E. coli was the most common cultured organism in those under 1 year. In neonates, E. coli accounted for 63.0% of cases. Group B Streptococcus (GBS) is regularly cited as the most common cause of meningitis in neonates and the lack of cases in this study may be attributed to high rates of prophylactic intrapartum antibiotics administered in Australia (6,14). Some of these neonates may have not been treated at QCH and instead treated in their local neonatal intensive care unit (NICU), hence not being captured in this study. Reducing rates of Haemophilus influenzae type B (Hib) have been seen worldwide due to widespread and effective immunisation programs (15,16). In keeping with Australia’s high rate of immunisation (8), no children were diagnosed with meningitis secondary to Hib. The incidence of SNHL was 5.3%, similar to that recently reported in New Zealand and the UK (17,18).
When considering whether all children received audiology follow up, 80.7% were known to audiology with 71.9% attending and completing formal audiology assessment. This does not account for those that chose to pursue audiology outside of the public health sector, thus the follow up rate was likely marginally higher than known. The wide-ranging time for referral (0 to 97 days) and varied time to review (8 to 304 days) demonstrates the lack of standardised referral guideline in Queensland for these children. Those children waiting such extended time for audiology assessment had increased risk of missing the cochlear implant window should they have had SNHL and subsequent early development of labyrinthitis ossificans.
On review of local and international guidelines for audiology assessment post bacterial meningitis, the UK National Institute for Health and Care Excellence (NICE) guidelines (13) provide a simple evidence-based guide to review. The guidelines recommend firstly offering formal audiological testing, as soon as possible (prior to discharge or within 4 weeks of being “fit to test”. Secondly, all children should be reviewed by a paediatrician, including their hearing test result, within 4–6 weeks of discharge. And finally, if severe or profound hearing loss is identified, urgent assessment for cochlear implant candidacy should occur (13). The New Zealand health service guidelines suggest more timely review, recommending audiology prior to discharge or within 7–10 days of discharge. Again “urgent” assessment for cochlear implantation in those with severe or profound hearing loss is recommended (12). More locally, the Royal Children’s Hospital in Melbourne recommends formal audiology assessment 6–8 weeks after discharge or earlier if concerns for hearing loss are raised (11).
The frequency and duration of repeat testing varies among guidelines with inconsistent evidence to support their development. Repeat testing is not addressed in the NICE Guidelines, whereas New Zealand specifically cites the lack of evidence and recommends repeat testing as optional if hearing is normal at the first assessment. Consistent with our findings, a review of over 200 adult and paediatric patients in the Netherlands found that only those with hearing loss evident on the first post-meningitis audiogram showed any deterioration in status with time (19). Richardson reported similar findings 20 years earlier (20). This does however conflict with other studies suggesting a rare risk of decline in sensorineural thresholds over time (21-23).
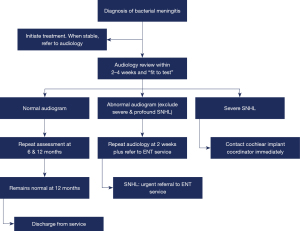
As a result of the above data and review of local and international surveillance guidelines, QCH has recommended that all children with a diagnosis or high clinical suspicion of bacterial meningitis should undergo formal audiology testing within 2–4 weeks of diagnosis and any referral be triaged to be seen within 30 days. Acknowledging the potentially life-threatening complications of meningitis and difficulties in conducting an auditory brainstem response (ABR) testing within an intensive care environment, this 2–4 week window must also consider that the child is “fit to test”. Importantly, audiology referral and review should not be delayed until after discharge or on first paediatric outpatient review. Inclusion of audiology review as part of an Allied Heath discharge checklist should be considered to reduce the risk of potential failure to test complex patients. Whilst no children in this study were found to have new SNHL on subsequent audiology if their first test was normal, we have recommended repeat audiology at 6 and 12 months. The rationale for this is multifactorial. A small number of studies have identified new progressive SNHL after meningitis in children with initial normal audiology and these children are also considered at risk for developmental delays and cognitive impairment (7,21,23,24). Should hearing loss of any degree be confirmed on initial testing, urgent referral should be made to an ENT surgeon and repeat audiology should occur at 2 weeks to qualify the loss and identify any rapidly progressive change. Importantly, if the initial audiogram suggests severe or profound SNHL, the audiologist should make immediate contact with the cochlear implant coordinator to expedite the child’s care (Figure 2).
One limitation of this study is the failure to capture use of dexamethasone early in the treatment paradigm. The use of systemic corticosteroids in bacterial meningitis has been well established to reduce hearing loss in bacterial meningitis (5,25). The use of dexamethasone is considered standard of care in Queensland and thus it can be assumed that the majority of non-neonatal cases were treated accordingly. Other limitations include the lack of data on patients solely treated in NICUs and the relatively small sample size, attributable to the small population of Queensland and thus the limited number of patients experiencing hearing loss. It is not powered to assess the rate and severity of hearing loss linked to Streptococcal infection nor the subsequent outcomes of cochlear implantation. A multicentre study would be required to address this limitation. With the implementation of new referral and surveillance guidelines at QCH, a prospective study to examine compliance, effectiveness and accessibility would further add to the literature in this area.
Conclusions
The findings of this study support those published in recent literature from developed countries, with Streptococcus sp. being associated with SNHL. It demonstrates wide variation in time to referral and review for audiology, highlighting a lack of awareness of the urgency for assessment and the implications of possible cochlear implantation. A larger, multicentre study would need to be conducted to draw conclusions on rate and success of cochlear implantation post bacterial meningitis.
On review of the current literature, local and international guidelines, we have developed a relevant and achievable referral pathway for clinicians to follow for children with bacterial meningitis. Re-review will be required in the future to determine if this guideline has been implemented and resulted in improved patient outcomes. We acknowledge that guidelines exist in other states of Australia and internationally. The authors would support a comprehensive Australia wide review with a view to producing a national referral guideline for Australia and New Zealand.
Acknowledgments
The authors would like to acknowledge all members of the Otolaryngology and Audiology Departments at Queensland Children’s Hospital, in particular, the members of the multidisciplinary implant team.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/10.21037/ajo-23-45/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-45/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-45/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was gained from Children’s Health Queensland Human Research Ethics Committee (project number 87811). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Manolidis S, Johnson R. Do corticosteroids prevent hearing loss in pediatric bacterial meningitis? An analysis of the evidence. Ear Nose Throat J 2006;85:586-92. [Crossref] [PubMed]
- Nadol JB Jr. Hearing loss as a sequela of meningitis. Laryngoscope 1978;88:739-55. [Crossref] [PubMed]
- Snoek L, Gonçalves BP, Horváth-Puhó E, et al. Short-term and long-term risk of mortality and neurodevelopmental impairments after bacterial meningitis during infancy in children in Denmark and the Netherlands: a nationwide matched cohort study. Lancet Child Adolesc Health 2022;6:633-42. [Crossref] [PubMed]
- Adil SM, Hodges SE, Charalambous LT, et al. Paediatric bacterial meningitis in the USA: outcomes and healthcare resource utilization of nosocomial versus community-acquired infection. J Med Microbiol 2021; [Crossref] [PubMed]
- Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2015;2015:CD004405. [PubMed]
- Agrawal S, Nadel S. Acute bacterial meningitis in infants and children: epidemiology and management. Paediatr Drugs 2011;13:385-400. [Crossref] [PubMed]
- Zainel A, Mitchell H, Sadarangani M. Bacterial Meningitis in Children: Neurological Complications, Associated Risk Factors, and Prevention. Microorganisms 2021;9:535. [Crossref] [PubMed]
- Department of Health and Aged Care, Commonwealth of Australia [Internet]. 2023 [cited 2023 Aug 22]. Childhood immunisation coverage data (PHN and SA3). Available online: https://www.health.gov.au/resources/collections/childhood-immunisation-coverage-data-phn-and-sa3
- Tinling SP, Colton J, Brodie HA. Location and timing of initial osteoid deposition in postmeningitic labyrinthitis ossificans determined by multiple fluorescent labels. Laryngoscope 2004;114:675-80. [Crossref] [PubMed]
- Philippon D, Bergeron F, Ferron P, et al. Cochlear implantation in postmeningitic deafness. Otol Neurotol 2010;31:83-7. [Crossref] [PubMed]
- Royal Children’s Hospital [Internet]. 2020 [cited 2023 Feb 5]. Clinical Practice Guideline: Meningitis and Encephalitis. Available online: https://www.rch.org.au/clinicalguide/guideline_index/Meningitis_encephalitis/
- Revised Audiology Guidelines for babies with meningitis [Internet]. 2021 Aug [cited 2023 Sep 17]. Available online: https://www.nsu.govt.nz/publications/revised-audiology-guidelines-babies-meningitis
- Meningitis (bacterial) and meningococcal septicaemia in under 16s: recognition, diagnosis and management Clinical guideline [Internet]. 2022. Available online: www.nice.org.uk/guidance/cg102
- Moorhead R, Daley AJ, Lee LY, et al. Compliance with screening for and recommended management of maternal group B streptococcus carriage in pregnancy. Aust N Z J Obstet Gynaecol 2019;59:837-42. [Crossref] [PubMed]
- Swartz MN. Bacterial meningitis--a view of the past 90 years. N Engl J Med 2004;351:1826-8. [Crossref] [PubMed]
- Oordt-Speets AM, Bolijn R, van Hoorn RC, et al. Global etiology of bacterial meningitis: A systematic review and meta-analysis. PLoS One 2018;13:e0198772. [Crossref] [PubMed]
- Drake R, Dravitski J, Voss L. Hearing in children after meningococcal meningitis. J Paediatr Child Health 2000;36:240-3. [Crossref] [PubMed]
- Fortnum H, Davis A. Hearing impairment in children after bacterial meningitis: incidence and resource implications. Br J Audiol 1993;27:43-52. [Crossref] [PubMed]
- Rodenburg-Vlot MBA, Ruytjens L, Oostenbrink R, et al. Repeated Audiometry After Bacterial Meningitis: Consequences for Future Management. Otol Neurotol 2018;39:e301-6. [Crossref] [PubMed]
- Richardson MP, Reid A, Tarlow MJ, et al. Hearing loss during bacterial meningitis. Arch Dis Child 1997;76:134-8. [Crossref] [PubMed]
- Jensen ES, Cayé-Thomasen P, Bodilsen J, et al. Hearing Loss in Bacterial Meningitis Revisited-Evolution and Recovery. Open Forum Infect Dis 2023;10:ofad056. [Crossref] [PubMed]
- Brookhouser PE, Worthington DW, Kelly WJ. Fluctuating and/or progressive sensorineural hearing loss in children. Laryngoscope 1994;104:958-64. [Crossref] [PubMed]
- Woolley AL, Kirk KA, Neumann AM Jr, et al. Risk factors for hearing loss from meningitis in children: the Children's Hospital experience. Arch Otolaryngol Head Neck Surg 1999;125:509-14. [Crossref] [PubMed]
- Christie D, Rashid H, El-Bashir H, et al. Impact of meningitis on intelligence and development: A systematic review and meta-analysis. PLoS One 2017;12:e0175024. [Crossref] [PubMed]
- Wang Y, Liu X, Wang Y, et al. Meta-analysis of adjunctive dexamethasone to improve clinical outcome of bacterial meningitis in children. Childs Nerv Syst 2018;34:217-23. [Crossref] [PubMed]
Cite this article as: Henrys C, Lloyd G, Lynch A. Recommendations for audiometric follow up post paediatric bacterial meningitis based on an Australian experience: a retrospective cross-sectional study. Aust J Otolaryngol 2024;7:9.


