
Sensorineural hearing loss in otic capsule-sparing petrous temporal bone fractures: an under-recognised phenomenon
Introduction
Traditional classification of petrous temporal bone (PTB) fractures relied upon radiologic findings of a transverse or longitudinal fracture line. However, a more recent classification system has categorised fractures as either otic capsule-sparing or violating, which provides for clinically prognostic data regarding sequelae such as facial nerve palsy, cerebrospinal fluid leakage, conductive and sensorineural hearing losses (SNHLs), and vestibular dysfunction (1,2). Part of the PTB, the otic capsule ensconces the membranous labyrinth of the inner ear; its contents include the cochlea, vestibule, and three semicircular canals. While it is well recognised that fractures through the capsule (otic capsule-violating) are associated with increased risk of SNHL (1,3), data examining the hearing outcomes of those with otic capsule-sparing fractures is sparse. We seek to identify whether otic capsule-sparing PTB fractures contribute to hearing loss; in light of the known correlation between hearing impairment and worsened quality of life, and given the protracted natural history of SNHL in general (4), early assessment and intervention provides an opportunity to ameliorate outcomes. We present this article in accordance with STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-44/rc).
Methods
Design & setting
We performed an observational analytic study, reviewing cases referred to the otolaryngology service at a level one trauma service in Melbourne, Australia over a four-year period from September 2018 to August 2022. Cases were found via ear, nose, and throat (ENT) unit audit data, and 207 records were reviewed for inclusion using hospital electronic medical records.
Participants & main outcome measures
We used computed tomography (CT) reports to identify unilateral, otic capsule-sparing fractures; images were also reviewed by an independent radiologist to determine the concordance of findings with those reported. This radiologist was aware that our patient population consisted of those with PTB fractures, but was blinded to the content of each report regarding side and location of fracture. Where discrepancies occurred, scans were re-reviewed by the original reporting radiologist and the study radiologist until agreement was reached.
Patients with prior known hearing impairment or without available post-injury audiogram results were excluded. Given the emergent nature of patient presentations, and in the absence of referral letters or other clinical corroboration, we relied heavily upon self-reported medical histories when determining whether patients had a pre-existing hearing impairment; as such, patients who reported generally poor hearing pre-injury were excluded from further analysis, along with those who espoused any of the diagnoses listed under the Disorders with Hearing Impairment in the International Classification of Diseases for Mortality and Morbidity Statistics, 11th Revision. Audiogram results were only included if performed within 365 days of injury. Other relevant retrospective patient demographic and clinical data were then obtained from medical records, and differences in four-frequency bone conduction threshold (BCT) (at 0.5, 1, 2, and 4 kHz) between side of PTB fracture and unaffected side were calculated. The World Health Organisation uses the average of conduction thresholds at 0.5, 1, 2, and 4 kHz to determine degree of hearing impairment, with losses >25 dB commonly used to denote a clinically significant hearing loss in the literature (5,6).
Statistical methods
Data analysis was performed with GraphPad Prism version 9.4.1 (GraphPad Software, San Diego, CA, USA). We assessed the normality of variables using D’Agostino & Pearson testing. Given the non-parametric distribution of all analysed datasets, we generally employed the Wilcoxon test when comparing two paired variables, the Mann-Whitney test for unpaired variables, and extracted Spearman’s rank correlation coefficient in cases of non-parametric correlation. Non-parametric datasets of sufficient size were also analysed using the t-test (paired or unpaired), in accordance with the central limit theorem. P values <0.05 in two-tailed tests were considered statistically significant.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Alfred Health (No. 630/22) prior to data collection and individual consent for this retrospective analysis was waived.
Results
Of 122 unilateral otic capsule-sparing PTB fractures, the average age of those affected was 43.9 years, and 86.1% were male (Table 1). One participant (0.82%) had known bone density disease, specifically osteoporosis; this is well below the general rate of osteoporosis in the total Australian population (3.8%) (7), but correlates with the young average age of included individuals in this study.
Table 1
| Variables | Values |
|---|---|
| Age (years), mean [range] | 43.9 [16–85] |
| Sex | |
| Male | 105 (86.1) |
| Female | 17 (13.9) |
| Pre-existing hearing loss | |
| Yes | 1 (0.82) |
| No | 121 (99.2) |
| Known bone density disease | |
| Yes | 1 (0.82) |
| No | 121 (99.2) |
| Fracture site | |
| Right | 64 (52.5) |
| Left | 58 (47.5) |
| Polytrauma (≥1 injury outside head) | |
| Yes | 41 (33.6) |
| No | 81 (66.4) |
| Intracranial haemorrhage | |
| Yes | 99 (81.1) |
| No | 23 (18.9) |
Values are given as number (percentage) unless otherwise indicated.
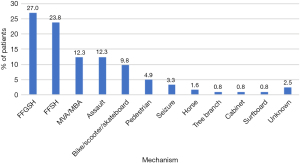
All known presentations occurred secondary to trauma (Figure 1). Twenty-seven percent of cases were caused by a fall from height greater than standing, as opposed to 23.8% by a fall from standing height. Car and motorbike accidents resulted in 12.3% of cases, as did head injuries from alleged assaults. Head injury sustained while riding bicycles, scooters or skateboards comprised a further 9.8% of cases, and pedestrian accidents another 4.9%. Seizures (3.3%), and head strikes from horses (1.6%), tree branches (0.8%), cabinets (0.8%), and surfboards (0.8%) were rarer causes of injury. Three PTB fractures resulted from unknown mechanisms. Despite some presentations being associated with significant polytrauma, no deaths occurred in the inpatient setting. There was a median duration of 23 (range, 0–305) days to audiometry.
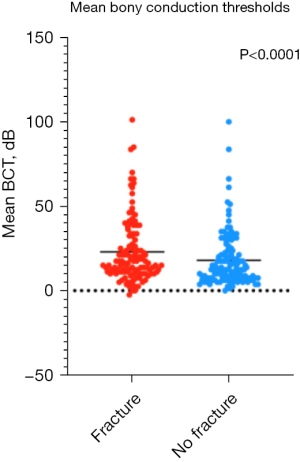
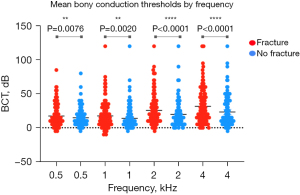
Thirty-three individuals (27.0%) had a mean BCT >25 dB in their PTB fracture-affected ear, as compared to 26 non-fractured ears (21.3%) (P=0.32). Notably, mean BCT was 5.05 dB worse in ears with PTB fractures (Figure 2) (95% CI: 3.2–6.9, P<0.0001). Of this cohort, 20.5% had a 10 dB or greater BCT deficit in their fracture-affected ear. Discrepancy in hearing between fracture-affected and unaffected ears increased with higher frequencies, with an average 2.6 dB deficit at 0.5 kHz, 3.5 dB at 1 kHz, 5.8 dB at 2 kHz, and 8.3 dB at 4 kHz (Figure 3). In fractured ears, hearing was worse at higher frequencies (median 10dB across the average of 2 and 4 kHz) than at lower frequencies (median 5 dB across the average of 0.5 and 1 kHz). This trend was even more pronounced in those with a 10 dB or greater deficit in their fractured ear relative to its non-fractured pair, with a median difference of 20 dB (P=0.0003) between higher (2 and 4 kHz) and lower (0.5 and 1 kHz) frequency BCTs.
Within both fractured and non-fractured subgroups, age was correlated with worsened hearing (Spearman’s r value =0.69 and 0.63 respectively, P<0.0001). Sex was not associated with BCT; neither were polytrauma or presence of intracranial haemorrhage. Hearing of paired fractured and unfractured ears is highly correlated (Spearman’s r value =0.78, P<0.0001).
Six patients (4.9%) had tympanic membrane (TM) perforation of their fracture-affected side. BCT of perforated ears was 34.0 dB on average, compared to 22.4 dB for fracture-affected ears with an intact TM, a mean difference of 11.5 dB (95% CI: −4.15–27.2, P=0.15). There were however no statistically significant differences in hearing between perforated and intact TMs at 0.5 kHz (P=0.85), 1 kHz (P=0.13), and 2 kHz (P=0.06). There was a difference at 4 kHz, with mean hearing 25.5 dB worse in perforated ears than those without perforation (95% CI: 4.45–46.5, P=0.04). Furthermore, when accounting for the BCT difference between paired fractured and unfractured ears, TM perforation significantly worsened overall hearing (P=0.035).
Eighty-six patients (70.5%) had haemotympanum on their injured side. Presence of haemotympanum did not significantly affect BCT in fractured ears (mean 22.9 vs. 23.3 dB, P=0.91). This result did not reach significant when adjusted according to the difference in BCT between paired ears (P=0.91).
Fifteen patients (12.3%) received corticosteroids throughout their admission; in 13 cases, documented facial paralysis was the primary indication for use of oral or parenteral corticosteroid. Administration of corticosteroids was not found to significantly alter BCT, with a mean BCT of 21.4 dB in those given steroids and a BCT of 23.2 dB in those who did not receive steroids (P=0.72). Adjustment for BCT difference between paired fractured and unfractured ears did not affect the significance of this result (P=0.68). There was no correlation between severity of facial nerve palsy and hearing loss, based either on initial (r=−0.05, P=0.88) or maximal (r=0.11, P=0.67) facial nerve deficit.
Where SNHL was found (25.9%), magnetic resonance imaging (MRI) to exclude acoustic neuroma or other retrocochlear pathology was only performed in 14.3% (three patients); none found retrocochlear pathology that would provide an alternate cause for asymmetric SNHL.
Discussion
Key results & comparison to other studies
In our cohort, 122 of 207 patients (58.9%) had PTB fractures that were unilateral and otic capsule-sparing. We excluded 20 bilateral fractures (9.7%), eight otic capsule-violating fractures (3.9%), 12 patients with pre-existing hearing loss (5.8%), and 45 patients without audiometry (21.7%). Our rate of otic capsule-violating fractures, comprising 3.9% of presentations, was lower than the 20% reported by Little and Kesser in their American study examining the predictive abilities of the otic capsule classification system (1), but was similar to those reported by Rafferty et al. (7%), Dahiya et al. (5.6%) and Brodie and Thompson (2.5%) (3,8,9). Little and Kesser’s rate of bilateral fractures (3.6%) was below our own (9.7%); however, their sample size only totalled 30 patients (1). Motor vehicle accidents and falls were uniformly the most common traumatic precipitants (1,3), reflective of the high degree of force required to disrupt the temporal bone. Young median age and a male preponderance was noted in our patient population, a common trend in trauma case series (1,3,8,10). Our rate of death, at 0%, was lower than that noted in other studies (7.9–9.2%) (10,11); this likely relates to our retrospective study design, given we excluded all cases that did not survive to audiometry. In Dahiya et al.’s study, 14.1% of otic capsule-sparing PTB fractures were associated with profound SNHL (3). Rafferty et al. report a 7% rate of SNHL in otic capsule-sparing PTB fracture (8); notably, both studies do not provide audiometric definitions for documented hearing losses, making direct comparison to our study challenging.
Antoniades et al.’s study of hearing outcomes following PTB fractures found that while CHL improved over 18 months post-injury, SNHL was more recalcitrant; 54.5% of CHL cases recovered normal hearing with time, as opposed to 12.5% of SNHL patients (4). Other studies similarly report positive outcomes for those with CHL, and persistent deficits for those with SNHL (8,10). Worsened hearing is correlated with worsened quality of life (4); this is concerning for those with traumatic brain injuries, who are at ongoing increased risk of developing SNHL, even at a delay of months or years (12). There is a dearth of published literature to describe the exact threshold in dB at which hearing loss is noticed, or has an impact on quality of life. In fact, some studies show poor correlation between patient reported burden, via the Hearing Handicap Inventory or similar, and degree of audiometric deficit (13-15). We arbitrarily assigned a significance of 10 dB between ears to examine certain outcomes; 20.5% of our cohort met this threshold. In these patients, more steeply down-sloping losses were noted than in the general population, with 2 and 4 kHz an average 20 dB below hearing thresholds at 0.5 and 1 kHz, against 10 dB in those with milder overall losses.
The causes of SNHL in otic capsule-sparing PTB fractures may be multifaceted. Mun et al.’s study of 34 patients with otic capsule-sparing PTB fractures examined the shortest measurable distance between the fracture line and the otic capsule on CT (16). They found that as fractures approached the otic capsule they were correlated with increasing severity of SNHL (16). Injury to central auditory pathways, cochlear disruption due to barotrauma, shearing of the auditory nerve with forceful brain injury, disruption of the membranous labyrinth, avulsion or trauma to the cochlear nerve, interruption of the cochlear blood supply, and perilymphatic fistula all represent possible contributing mechanisms to SNHL (12,17). Endolymphatic hydrops is also postulated to result from obstruction of the endolymphatic duct by displaced temporal bone (17). Traumatic brain injury has been linked to loss of outer hair cells of the cochlea, loss of spiral ganglion cells, and cochlea haemorrhage, even in the absence of skull fracture (18). Where the auditory epithelium is damaged, permanent scar tissue forms that is unable to regenerate replacement hair cells (19). Increasing average losses with increasing frequencies of sound imply damage to cochlear structures such as the outer hair cells. Noise-induced hearing loss will follow the same pattern, with increased deficits at increasing frequencies and a so-called “noise notch” at 4 kHz; it is difficult to determine the influence of this on our cohort given we did not examine high frequency thresholds (6 or 8 kHz). It is worth noting that the Carhart effect, in those with co-existing CHL, will raise BCTs, particularly at high frequencies (20), and that we did not exclude such cases from our data. Intracranial haemorrhage was not associated with worsening hearing, which again implies central auditory injury is of secondary importance; whether this may change over time, in accordance with Shangkuan et al.’s results (12), is not something we were able to assess with our data.
It is interesting to note that, while haemotympanum did not affect mean BCT, those with perforated TMs did have worse hearing losses via this assessment. In those with normal external and middle ear function, BCT principally assesses SNHL, rather than conductive pathways, and hence on superficial consideration should have little relationship to TM function. We posit that TM perforation is correlated with higher energy and louder trauma which may transmit greater damage to other inner ear structures.
We did not find any differences in hearing outcomes between those receiving steroids and those who did not (mean BCT 21.4 vs. 23.2 dB, P=0.72). The use of steroids in SNHL has been better described in patients with sudden SNHL, where the most common aetiology is postulated to be an inflammatory response (21). The hearing loss in patients with PTB fractures is suspected to have a vastly different range of aetiologies, as described above. While steroids may reduce post traumatic swelling, their influence on hearing loss caused by avulsion, shearing or damage to blood supply or hair cells is unknown.
Limitations
The retrospective nature of our data introduces several methodological weaknesses. Firstly, the choice of unfractured fellow ears as a control group; paired ears of a single individual are evidently related, and must both suffer a degree of injury in the trauma causing unilateral PTB fracture, which may or may not influence the hearing of the unfractured ear. However, in the absence of pre-injury audiograms, pairing of fellow ears was considered the most appropriate comparison of pre-morbid hearing available. Furthermore, the significant heterogeneity in the timing of post-injury audiograms introduces further confounding, in light of the known dynamic rate of SNHL development post-injury (4,8-10,12). Nonetheless, our data provides several hypotheses that a prospective study should more rigorously interrogate. Future studies that are able to more comprehensively assess duration and doses of corticosteroid administered to those with otic capsule-sparing PTB fractures, and to sequentially perform audiometry at specified intervals before, during, and after treatment, would be of benefit in substantiating our data.
Conclusions
Our study is the first to quantify the mean difference in hearing between ears in cases of unilateral otic capsule-sparing PTB fracture. Otic capsule-sparing PTB fractures are associated with SNHL, particularly at high frequencies. Patients can be reassured that in many cases, this hearing loss is mild, and unlikely to be appreciably noticed. There is a small group of patients with a more significant SNHL which may benefit from rehabilitation. Consideration of early audiometry in the acute setting, and prescription of high-dose steroids as required, warrants further exploration as a means of improving final hearing outcomes in otic capsule-sparing PTB fractured ears.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/10.21037/ajo-23-44/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-44/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-44/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-44/coif). F.C.E.H. received support from CochlearTM to attend cochlear conferences. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Alfred Health (No. 630/22) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Little SC, Kesser BW. Radiographic classification of temporal bone fractures: clinical predictability using a new system. Arch Otolaryngol Head Neck Surg 2006;132:1300-4. [Crossref] [PubMed]
- Morales Puebla JM, López Juanes N, Varo Alonso M, et al. Clinical-radiological Correlation in Temporal Bone Fractures. Acta Otorrinolaringol Esp (Engl Ed) 2021;72:295-304. [Crossref] [PubMed]
- Dahiya R, Keller JD, Litofsky NS, et al. Temporal bone fractures: otic capsule sparing versus otic capsule violating clinical and radiographic considerations. J Trauma 1999;47:1079-83. [Crossref] [PubMed]
- Antoniades E, Psillas G, Polyzoidis K, et al. Patient-Assessed Outcomes following Temporal Bone Fractures. Diagnostics (Basel) 2022;12:547. [Crossref] [PubMed]
- Mathers C, Smith A, Concha M. Global burden of hearing loss in the year 2000. Global Burden of Disease 2000;18:1-30.
- Vermiglio AJ, Griffin S, Post C, et al. An Evaluation of the World Health Organization and American Medical Association Ratings of Hearing Impairment and Simulated Single-Sided Deafness. J Am Acad Audiol 2018;29:634-47. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. Osteoporosis. AIHW. Updated August, 2020. Accessed November 21, 2022. Available online: https://www.aihw.gov.au/reports/chronic-musculoskeletal-conditions/osteoporosis/contents/about
- Rafferty MA, Mc Conn Walsh R, Walsh MA. A comparison of temporal bone fracture classification systems. Clin Otolaryngol 2006;31:287-91. [Crossref] [PubMed]
- Brodie HA, Thompson TC. Management of complications from 820 temporal bone fractures. Am J Otol 1997;18:188-97. [PubMed]
- Ricciardiello F, Mazzone S, Longo G, et al. Our Experience on Temporal Bone Fractures: Retrospective Analysis of 141 Cases. J Clin Med 2021;10:201. [Crossref] [PubMed]
- Schubl SD, Klein TR, Robitsek RJ, et al. Temporal bone fracture: Evaluation in the era of modern computed tomography. Injury 2016;47:1893-7. [Crossref] [PubMed]
- Shangkuan WC, Lin HC, Shih CP, et al. Increased long-term risk of hearing loss in patients with traumatic brain injury: A nationwide population-based study. Laryngoscope 2017;127:2627-35. [Crossref] [PubMed]
- Matthews LJ, Lee FS, Mills JH, et al. Audiometric and subjective assessment of hearing handicap. Arch Otolaryngol Head Neck Surg 1990;116:1325-30. [Crossref] [PubMed]
- Newman CW, Jacobson GP, Hug GA, et al. Perceived hearing handicap of patients with unilateral or mild hearing loss. Ann Otol Rhinol Laryngol 1997;106:210-4. [Crossref] [PubMed]
- Weinstein BE, Ventry IM. Audiometric correlates of the Hearing Handicap Inventory for the elderly. J Speech Hear Disord 1983;48:379-84. [Crossref] [PubMed]
- Mun SK, Oh KH, Hong YH, et al. Using temporal bone computed tomography to predict sensorineural hearing loss in otic capsule-sparing temporal bone fracture. Injury 2017;48:2879-83. [Crossref] [PubMed]
- Rizvi SS, Gibbin KP. Effect of transverse temporal bone fracture on the fluid compartment of the inner ear. Ann Otol Rhinol Laryngol 1979;88:741-8. [Crossref] [PubMed]
- Uchiyama M, Monsanto RDC, Sancak IG, et al. Temporal Bone Pathology Secondary to Head Trauma-A Human Temporal Bone Study. Otol Neurotol 2021;42:e1152-9. [Crossref] [PubMed]
- Oesterle EC. Changes in the adult vertebrate auditory sensory epithelium after trauma. Hear Res 2013;297:91-8. [Crossref] [PubMed]
- Gatehouse S, Browning GG. A re-examination of the Carhart effect. Br J Audiol 1982;16:215-20. [Crossref] [PubMed]
- Wei BP, Stathopoulos D, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev 2013;2013:CD003998. [Crossref] [PubMed]
Cite this article as: Rowson AC, Yii MMX, Truong M, Kilby J, Tan H, Gan C, Webb H, Hill FCE. Sensorineural hearing loss in otic capsule-sparing petrous temporal bone fractures: an under-recognised phenomenon. Aust J Otolaryngol 2024;7:8.


