
The temporal association between new head & neck cancer diagnoses and local COVID-19 lockdown measures in Victoria: a population-based study
Introduction
The coronavirus disease (COVID-19) pandemic has had a significant impact on global health, with millions of cases and deaths reported worldwide (1). To prevent the spread of the virus, governments had implemented a range of measures, including lockdowns, social distancing, and restrictions on travel (2). These measures have had far-reaching consequences on healthcare delivery, with disruptions to cancer screening and diagnosis programs (3).
During the COVID-19 pandemic, Victoria’s experience was unique compared to the rest of world. Whilst, the overall case burden and the incidence of community transmission were significantly higher than in other states, the corresponding restriction were more imposing than other countries with much higher incidence of COVID-19. Victoria experienced two major lockdown periods during 2020, with a total of 154 days, March 30th till May 12th then July 8th till October 27th. Thus, the lockdown measures imposed locally make Victorian residents a special population in which to study the public health impact of COVID-19 lockdown. Given the importance of early detection and treatment in cancer management, the evaluation of oncological reporting during this period is an especially high priority (4). Australia-wide, cancer screening programs were disrupted to reduce the risk of COVID-19 transmission (5,6). Recommendations on limitation of aerosol generating procedures meant a reduction in the clinical and diagnostic use of fibreoptic endoscopy and endoscopic evaluations under anaesthetic. Similarly, in Europe, surveillance and screening programs were suspended or delayed preventing the spread of the virus (7). Patients were also more hesitant to seek medical attention for cancer symptoms due to concerns about contracting COVID-19 in healthcare settings (8).
Pathology notifications to the Victorian Cancer Registry (VCR) showed an overall reduction of 30% for the five most common cancers (colorectal, prostate, breast, melanoma, and lung) and an even greater reduction in reports for head and neck cancer (HNC) during the period between April and June 2020 (9). HNC is of particular concern during the pandemic, as many of its symptoms, such as a sore throat or difficulty swallowing, overlap with those of COVID-19 (10). This overlap can lead to misdiagnosis or delayed diagnosis of HNC, which could have significant consequences for patient outcomes. In addition, the pandemic has resulted in a reduction in the number of HNC surgeries performed (11). This delay in treatment can lead to cancer progression and worse patient outcomes.
HNC can progress quickly leading to a significant impact for individuals presenting with later stage disease and for the capacity of the health care system, including primary care to accommodate this. Jensen et al. found that in a cohort of Danish patients with head and neck squamous-cell carcinoma (SCC) awaiting radiotherapy, the majority developed significant signs of tumour progression within a 4-week period (7). Under normal circumstances, HNCs are triaged quickly, proceeding along an optimal care pathway from initial symptom presentation in a primary care setting, to specialist-led diagnosis and staging to definitive treatment. Several decision models were utilised during the COVID pandemic to guide this process (12).
The UK health system had a nationwide lockdown from March to June 2020. Those lockdown measures imposed dramatic changes on the delivery of medical services, leading to significant delays in presentations to primary care and consequently, reductions in urgent referrals for suspected cancer (10). An emergency department-based study noted a significant rise of newly diagnosed primary HNC and newly diagnosed HNC recurrence presentations post-lockdown (from June 2020 to November 2020), and this was in conjunction with a relative reduction in presentations through other clinical pathways. This proportionate shift towards acute presentations suggests that these may represent more advanced stage HNCs (13). These findings were substantiated in another European study, where a significant increase was found in the incidence of T3/4 stage HNCs from baseline level (pre-COVID pandemic) to late 2020 (8). Time from first symptoms to diagnosis was also significantly increased by approximately 3 weeks on average. In Texas, where COVID-19 related lockdown measures were lesser, a study noted that while time to diagnosis was not significantly delayed during the COVID era, there was a 25% reduction in newly diagnosed malignancies presented at their head and neck multidisciplinary meeting (MDM) from the previous year, indicative of the reduction in clinic scheduling and referrals. This corresponded to a significantly larger median primary tumour size and more advanced T stage for mucosal subsites compared to pre-COVID-19 numbers (14).
At present, there is a paucity of Australian studies on this subject. Given the findings from related international studies, and in the context of the strict lockdown measures enforced in Victoria, this research is a high priority. This study sought to determine whether the COVID-19 pandemic and the corresponding lockdown measures in Victoria have had a measurable impact on the rates of newly diagnosed HNC. We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-46/rc).
Methods
A retrospective review of HNCs diagnosed in Victorians between 2019 and 2020 was performed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of St Vincent’s Hospital Melbourne (LRR 005/22 – 79785) and individual consent for this retrospective analysis was waived.
Participants
HNC incidence data for Victorians diagnosed in 2019 and 2020 was obtained from the VCR, and extracted in December 2021. The VCR was established as a population-based registry in 1982, and covers the population of Victoria, comprising 6.6 million residents as of 2020. As mandated by the Improving Cancer Outcomes Act 2014, the VCR must be notified of all cancer diagnoses in Victoria. The rules for reporting multiple primary cancers of the International Agency for Research on Cancer (IARC) and the International Association of Cancer Registries (IACR) stipulate that only the first occurrence of a cancer of a particular type is counted for an individual. As of 2020, notifications were received from a multitude of Victorian centres, including 254 hospitals and 26 pathology laboratories.
Tumour morphology and topography are classified by their International Classification of Diseases (ICD-10) codes with major head and neck sites of lip, oral cavity, salivary glands, pharynx, nasal cavities, and larynx being utilised. The sites of oral cavity and pharynx have also been separated into the subsites shown in Table 1.
Table 1
| Site | ICD-10 codes | Year | Observed | Expected | Difference | SIR | 95% CI |
|---|---|---|---|---|---|---|---|
| Head and neck (all) | C00–C14, C30–C32 | 2019 | 1,135 | ||||
| 2020 | 1,111 | 1,167 | −56 | 95.2 | 89.7–100.9 | ||
| Lip | C00 | 2019 | 164 | ||||
| 2020 | 205 | 169 | 36 | 121.4 | 105.9–139.3* | ||
| Oral cavity (all) | C01–C06 | 2019 | 396 | ||||
| 2020 | 378 | 407 | −29 | 92.9 | 84–102.7 | ||
| Tongue | C01, C02 | 2019 | 261 | ||||
| 2020 | 233 | 268 | −35 | 87.0 | 76.5–98.9* | ||
| Gum | C03 | 2019 | 48 | ||||
| 2020 | 41 | 50 | −9 | 82.7 | 60.9–112.3 | ||
| Floor of mouth | C04 | 2019 | 28 | ||||
| 2020 | 35 | 29 | 6 | 121.0 | 86.9–168.6 | ||
| Other mouth | C05, C06 | 2019 | 59 | ||||
| 2020 | 69 | 61 | 8 | 113.8 | 89.9–144.1 | ||
| Salivary glands | C07, C08 | 2019 | 97 | ||||
| 2020 | 71 | 100 | −29 | 71.3 | 56.5–90* | ||
| Pharynx (all) | C09–C13 | 2019 | 269 | ||||
| 2020 | 257 | 276 | −19 | 93.1 | 82.4–105.2 | ||
| Oropharynx | C09, C10 | 2019 | 187 | ||||
| 2020 | 182 | 192 | −10 | 94.9 | 82.1–109.7 | ||
| Nasopharynx | C11 | 2019 | 47 | ||||
| 2020 | 36 | 48 | −12 | 74.8 | 54–103.7 | ||
| Hypopharynx | C12, C13 | 2019 | 35 | ||||
| 2020 | 39 | 36 | 3 | 107.7 | 78.7–147.4 | ||
| Nasal cavities | C30, C31 | 2019 | 68 | ||||
| 2020 | 66 | 70 | −4 | 93.9 | 73.8–119.6 | ||
| Larynx | C32 | 2019 | 129 | ||||
| 2020 | 123 | 133 | −10 | 92.4 | 77.4–110.2 |
Projected numbers are calculated from 2019 age and sex specific incidence data projected on 2020 population data. *, statistically significant. ICD-10, International Classification of Diseases; SIR, standardized incidence ratio (observed/expected); CI, confidence interval.
Quarterly estimated residential population data was obtained from the Australian Bureau of Statistics (15).
The 2 major lockdown periods during 2020, with a total of 154 days. The first lockdown was from 30th March through to the 12th May 2020, total of 43 days. The second lockdown period was then from 8th July till 27th October 2020.
Statistical analysis
Age-standardise rates (ASR; standardised to the World Segi Standard population) with 95% confidence intervals (CIs) were calculated for each quarter in 2019 and 2020 using the direct method (16,17). Age (in 5-year age groups) and sex specific cancer incidence rates extracted from 2019 data were applied to the 2020 population estimates to obtain the expected number of new cancer diagnoses in 2020. To compare the expected and observed number of new diagnoses, standardised incidence ratios (SIRs) with 95% CIs were calculated using the indirect method, for each quarter in 2020 for all HNCs combined as well as each of the subgroups (17). A SIR above 100 implies more diagnoses were observed than expected, whereas a SIR below 100 implies fewer diagnoses observed than expected.
Results
Overall HNCs
Among the 2,246 HNCs diagnosed in 2019 and 2020 (3.1% of all cancer diagnoses), those of the oral cavity (35%) were the most prevalent in Victoria, followed by the oropharynx (16%), and then larynx (11%).
HNC cases in 2020 were expected to increase to 1,167, approximately 3% up from 2019. In 2020, 1,111 new diagnoses were observed, approximately 5% below the projected value (SIR: 95; 95% CI: 88–101).
Breaking down to individual quarters, a statistically significant reduction of SIR (SIR: 85.5; 95% CI: 75.6–96.7) was demonstrated in quarter 2 as shown in Figure 1 which may represent the concern with HNC presenting symptoms overlapping with the public health advice of COVID symptoms and preceding the enforced lockdowns.
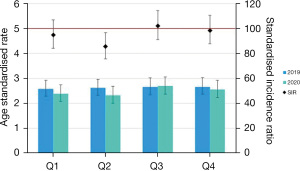
Oral cavity and oropharyngeal sites
Oral cavity and oropharyngeal subsites were the most commonly reported cases in the data overall, these demonstrated a reduction in observed rates (Table 1 and Figure 2) yet the subsite tongue was the only statistically significant SIR (SIR: 87.0; 95% CI: 76.5–98.9) when analysing the entire period (Q1–Q4) of 2020 (Table 2). Oral cavity when analysed shown in Figure 3 again demonstrates a similar trend to the overall head and neck data with a statistically significant (SIR: 80.1; 95% CI: 64.8–99.1) reduction during quarter 2 which coincides with the initial lockdown period in Victoria.
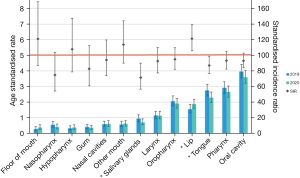
Table 2
| Subsite | 2019 | 2020 | SIR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| Observed | ASR | Observed | ASR | Expected | ||||
| All oral cavity (C01–C06) | ||||||||
| Q1 | 83 | 0.81 | 90 | 0.77 | 86 | 105.1 | 85.4–129.2 | |
| Q2 | 103 | 0.92 | 85 | 0.79 | 106 | 80.1 | 64.8–99.1* | |
| Q3 | 104 | 0.98 | 101 | 0.94 | 107 | 94.5 | 77.8–114.9 | |
| Q4 | 106 | 1.00 | 102 | 0.87 | 108 | 94.1 | 77.5–114.3 | |
| Q1–Q4 | 396 | 3.71 | 378 | 3.37 | 407 | 92.9 | 84.0–102.7 | |
| Tongue (C01, C02) | ||||||||
| Q1 | 56 | 0.58 | 51 | 0.44 | 58 | 88.4 | 67.2–116.3 | |
| Q2 | 69 | 0.67 | 54 | 0.52 | 71 | 76.1 | 58.3–99.3* | |
| Q3 | 63 | 0.63 | 64 | 0.61 | 65 | 99.0 | 77.5–126.5 | |
| Q4 | 73 | 0.71 | 64 | 0.57 | 75 | 85.8 | 67.1–109.6 | |
| Q1–Q4 | 261 | 2.59 | 233 | 2.14 | 269 | 87.0 | 76.5–98.9* | |
Quarters (Q1–Q4) represent 3 monthly periods. SIRs with 95% CI not including 100 are considered statistically significant. *, statistically significant. ASR, age-standardised rate; SIR, standardized incidence ratio (observed/expected); CI, confidence interval.

When stratified by sex there is a consistent reduction in observed rates from 2019 to 2020 through the progression of Q1–Q4 in males yet in females there is a rise in rates in Q3 (SIR: 105.7; 95% CI: 76.9–145.3) and Q4 (SIR: 111.0; 95% CI: 78.9–156.1) although the SIR is not statistically significant (Table 3).
Table 3
| Oral cavity (C01–C06) | 2019 | 2020 | SIR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| Observed | ASR | Observed | ASR | Expected | ||||
| Female | ||||||||
| Q1 | 18 | 0.30 | 36 | 0.58 | 19 | 193.8 | 139.8–268.6* | |
| Q2 | 37 | 0.61 | 33 | 0.54 | 38 | 86.5 | 61.5–121.7 | |
| Q3 | 35 | 0.60 | 38 | 0.65 | 36 | 105.7 | 76.9–145.3 | |
| Q4 | 29 | 0.48 | 33 | 0.47 | 30 | 111.0 | 78.9–156.1 | |
| Q1–Q4 | 119 | 1.99 | 140 | 2.24 | 123 | 114.4 | 96.9–135.0 | |
| Male | ||||||||
| Q1 | 65 | 1.35 | 54 | 0.98 | 67 | 80.6 | 61.7–105.3 | |
| Q2 | 66 | 1.26 | 52 | 1.05 | 68 | 76.6 | 58.4–100.6 | |
| Q3 | 69 | 1.39 | 63 | 1.25 | 71 | 89.0 | 69.5–113.9 | |
| Q4 | 77 | 1.57 | 69 | 2.03 | 79 | 87.8 | 69.3–111.1 | |
| Q1–Q4 | 277 | 5.57 | 238 | 5.31 | 285 | 83.7 | 73.7–95.0* | |
Q1–Q4 represent 3 monthly stratifications. ASR for 2019 and 2020 with SIR for the 2020 study period. SIRs with 95% CI not including 100 are considered statistically significant. *, statistically significant. ASR, age-standardised rate; SIR, standardized incidence ratio (observed/expected); CI, confidence interval.
Cancers of the lip increased from 164 in the year 2019, to 205 in the year 2020. This represented a 25% increase from 2019 and surpassed the projected number of cases by 21% (SIR: 121.4; 95% CI: 105.9–139.3). Floor of mouth cancers were a relatively smaller cohort (n=28 in 2019), but also saw an increase of 21% in 2020 above expected numbers but this was not statistically significant (SIR: 121.0; 95% CI: 86.9–168.6).
Salivary gland cancers demonstrated a statistically significant reduction for the Q1–Q4 period between 2019 and 2020 as demonstrated in Figure 2.
Discussion
Our data highlights a substantial change in the incidence of HNC detection in Victoria during the period of 2019 to 2020. HNC cases were expected to increase by approximately 3% from 2019 (to 1,167). Instead, that number was nearly 5% lower than this projection. This phenomenon is apparent in the subgroups of tongue and salivary gland.
An overall reduction in cases from expected is of great concern as the progression of HNC can be quick and change outcomes with stage progression. A systematic review and meta-analysis by Sharma et al. (2016) found that delays greater than 30 days in diagnosis to treatment of HNC were associated with higher mortality rates (18). The authors reported that patients with delayed diagnosis had a significantly increased risk of death compared to those with timely diagnosis, with a hazard ratio of 1.34 (95% CI: 1.20–1.49) (18).
In addition to increased mortality, delayed diagnosis can lead to more aggressive tumour biology and a higher risk of treatment-related complications. A systematic review by Schutte et al. (2020) found that delays in diagnosis and time to treatment of HNC were associated with poorer survival outcomes and in studies that demonstrated tumour growth potential treatment intensification and greater risk of poorer functional outcomes related to treatment (19).
Interestingly, this data demonstrates an increase in detection of lip cancer during COVID-19. There have been reports of an increase in the detection of lip cancer during the COVID-19 pandemic in some countries. An Italian study reported a 6.3% increase in the incidence of lip cancer during the period from 2016 to 2020 which included the pandemic (20). Several factors could contribute to the increased detection of lip cancer during the COVID-19 pandemic. One possible explanation is that the pandemic has led to changes in the use of personal protective equipment (PPE), which could also contribute to the increased detection of lip cancer. The prolonged use of face masks and other PPE could cause irritation to the lips, drawing attention to sores or ulcers that may have otherwise been ignored.
Stratification of the oral cavity data demonstrated differences between gender and rates over the 2019–2020 period (Table 3). There was an increase in observed rates between 2019–2020 in females, yet this was not statistically significant (SIR: 114.4; 95% CI: 96.9–135.0), whilst males had a reduction which was for Q1–Q4 statistically significant (SIR: 83.7; 95% CI: 73.7–95.0). The differences in rates of oral cavity cancer and gender may be explained by the rising incidence of oral cavity cancers in females, which has been reported in a multi-institutional study by Clark et al. in 2020 (21).
The literature indicates that the COVID-19 pandemic has led to a reduction in cancer detection rates due to decreased utilization of cancer screening services, disrupted healthcare systems, patient reluctance to seek medical attention, and diversion of resources to COVID-19 management (22). The impact of this is highlighted in our data, observed after only the first year of the COVID-19 pandemic in Victoria. Because state-based lockdown measures continued intermittently until the end of 2021, we expect that this phenomenon may be compounded further once 2021/2022 data is available.
Future directions should focus on mitigating the negative impact of the COVID-19 pandemic on cancer detection and management. This may involve implementing strategies to safely resume cancer screening services, such as telemedicine or modified clinic protocols including performing aerosol generating procedures and increasing public awareness about the importance of cancer screening and early detection. Additionally, efforts should be made to identify and address disparities in cancer care that have been exacerbated by the pandemic. Continued research is necessary to fully understand the long-term impact of COVID-19 on cancer outcomes and to develop effective strategies to minimize its negative effects.
Limitations
Cancer projections (expected incidence) are intended as a guide to assist in understanding from experience what future rates of cancer are expected to look like in Victoria. However, projection estimates should be used with caution, as they are influenced by the chosen reference period (2019 in our study). The projection estimates also do not consider any uncertainty around the future population estimates. These estimates of future population size by age and sex in this study were obtained from the Australian Bureau of Statistics (16).
Disease misclassification within a large dataset is possible, within this database, the risk is minimised by utilisation of medical coders classifying incoming data which is often cross referenced between both hospital and pathological notifications.
Staging data for head and neck cases was not available and the dataset that has been used for this study also did not involve 2021 or 2022 data to allow for further post lockdown evaluation and should be a future direction of this research.
Conclusions
Impacts of COVID-19 on healthcare provision and access have been multifaceted. The apparent reduction in HNC incidence in the population data from Victoria may have resulted from reduction in access to healthcare, apprehension in use of aerosol generating procedures earlier in the pandemic or reflect the dynamic migration of the Victorian population over the period of the COVID-19 pandemic. Potential delayed or missed diagnoses have significant impact on treatment and prognosis. The question remains the longer-term impact on the incidence of HNC and will a rebound of cases occurs within Victoria. Further analysis of this trend would be prudent to help inform future pandemic responses.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-46/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-46/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-46/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-46/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of St Vincent’s Hospital Melbourne (LRR 005/22 – 79785) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. COVID-19 Weekly Epidemiological Update. [Internet] 2021. [cited 2021 Feb 25]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update---23-february-2021
- European Centre for Disease Prevention and Control. Overview of the implementation of COVID-19 vaccination strategies and vaccine deployment plans in the EU/EEA. [Internet] 2021. [cited 2021 Feb 25]. Available online: https://www.ecdc.europa.eu/en/publications-data/overview-implementation-covid-19-vaccination-strategies-and-vaccine-deployment
- Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol 2020;21:750-1. [Crossref] [PubMed]
- Sankaranarayanan R, Ramadas K, Qiao YL. Managing the changing burden of cancer in Asia. BMC Med 2014;12:3. [Crossref] [PubMed]
- Australian Institute of Health and Welfare 2021. Cancer screening and COVID-19 in Australia. Cat. no. CAN 137. Canberra: AIHW.
- Australian Government Department of Health and Aged Care. Simulated impacts of COVID-19 scenarios on cancer screening – summary report. Available online: https://www.health.gov.au/resources/publications/simulated-impacts-of-covid-19-scenarios-on-cancer-screening-summary-report
- Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol 2007;84:5-10. [Crossref] [PubMed]
- Topf MC, Shenson JA, Holsinger FC, et al. Framework for prioritizing head and neck surgery during the COVID-19 pandemic. Head Neck 2020;42:1159-67. [Crossref] [PubMed]
- Te Marvelde L, Wolfe R, McArthur G, et al. Decline in cancer pathology notifications during the 2020 COVID-19-related restrictions in Victoria. Med J Aust 2021;214:281-3. [Crossref] [PubMed]
- Wilkie MD, Gaskell P, Hall B, et al. Emergency presentations of head and neck cancer: Our experience in the wake of the COVID-19 pandemic. Clin Otolaryngol 2021;46:1237-41. [Crossref] [PubMed]
- Bannister M, Vallamkondu V, Ah-See KW. Emergency presentations of head and neck cancer: a modern perspective. J Laryngol Otol 2016;130:571-4. [Crossref] [PubMed]
- de Almeida JR, Noel CW, Forner D, et al. Development and validation of a Surgical Prioritization and Ranking Tool and Navigation Aid for Head and Neck Cancer (SPARTAN-HN) in a scarce resource setting: Response to the COVID-19 pandemic. Cancer 2020;126:4895-904. [Crossref] [PubMed]
- Tevetoğlu F, Kara S, Aliyeva C, et al. Delayed presentation of head and neck cancer patients during COVID-19 pandemic. Eur Arch Otorhinolaryngol 2021;278:5081-5. [Crossref] [PubMed]
- Kiong KL, Diaz EM, Gross ND, et al. The impact of COVID-19 on head and neck cancer diagnosis and disease extent. Head Neck 2021;43:1890-7. [Crossref] [PubMed]
- Australian Bureau of Statistics. National, state and territory population methodology. June 2022. [accessed 11 May 2023]. Available online: www.abs.gov.au
- Segi M. Cancer mortality for selected sites in 24 countries 1950–1957. Sendai, Japan: Department of Public Health, Tohoku University School of Medicine; 1960.
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ 1987;1-406. [PubMed]
- Sharma S, Bekelman J, Lin A, et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with oropharyngeal squamous cell carcinoma. Oral Oncol 2016;56:17-24. [Crossref] [PubMed]
- Schutte HW, Heutink F, Wellenstein DJ, et al. Impact of Time to Diagnosis and Treatment in Head and Neck Cancer: A Systematic Review. Otolaryngol Head Neck Surg 2020;162:446-57. [Crossref] [PubMed]
- Mangone L, Mancuso P, Bisceglia I, et al. The epidemiology of oral cancer during the COVID-19 pandemic in Northern Italy: Incidence, survival, prevalence. Front Oral Health 2022;3:982584. [Crossref] [PubMed]
- Satgunaseelan L, Allanson BM, Asher R, et al. The incidence of squamous cell carcinoma of the oral tongue is rising in young non-smoking women: An international multi-institutional analysis. Oral Oncol 2020;110:104875. [Crossref] [PubMed]
- Alkatout I, Biebl M, Momenimovahed Z, et al. Has COVID-19 Affected Cancer Screening Programs? A Systematic Review. Front Oncol 2021;11:675038. [Crossref] [PubMed]
Cite this article as: Hart C, Manji J, Te Marvelde L, Dharmawardana N, Dixon B. The temporal association between new head & neck cancer diagnoses and local COVID-19 lockdown measures in Victoria: a population-based study. Aust J Otolaryngol 2024;7:2.


