A randomised placebo-controlled trial of topicalization for flexible nasal endoscopy
Introduction
Flexible nasal endoscopy (FNE) is a routine examination in any otolaryngology department. It is an important part of the assessment of the nasal airway, pharynx, and larynx. Many otolaryngologists are concerned that this examination can be a significant source of discomfort for the patient, especially those patients who require regular screening with FNE and therefore try to reduce this discomfort. There are many approaches to topicalization or lubrication in preparation of FNE which include: lubrication with saline, water-soluble lubricating gel, or topical anaesthesia with a lignocaine-based spray such as Co-phenylcaine Forte (Flo, ENT Technologies; Victoria, Australia).
Co-phenylcaine Forte spray is a combination of lignocaine hydrochloride, a topical anaesthetic, and phenylephrine hydrochloride, a vasoconstrictor and nasal decongestant. The degree to which topical vasoconstrictors alone affect patient discomfort is still unknown. Logically, increasing the nasal aperture by reducing congestion with a vasoconstrictor would improve ease of examination with a nasal endoscope. The efficacy/utility of decongestant sprays has been quite varied in the literature—some studies have shown no benefit to decongestant use in comparison with placebo, while other studies have shown decongestant use to be inferior to placebo due to the poor taste attributed from the patient’s perspective (1-3). These studies focused on the patient experience rather than the clinician’s experience and did not record the ease with which the scope was passed or the quality of the view.
Further research is needed to confirm or refute the efficacy of lubricating agents, and the impact on examiner experience. There are also limited studies which consider the impact of examiner experience level on patient outcomes. This study aims to evaluate both patient and examiner experiences during FNE as a double-blinded randomized controlled trial (RCT) with four different examination adjuncts: no treatment vs. saline (placebo) vs. Co-phenylcaine Forte (local anaesthetic and decongestant) vs. water-soluble gel lubricant. Patient outcomes investigated were pain, discomfort, taste, and repeatability recorded through visual analogue scale (VAS) scores. Examiner outcomes investigated pertained to ease of passing scope, and quality of view. The effect of examiner experience level was also investigated ranging from resident medical officers (RMOs), principal house officers (PHOs), accredited registrars (REGs), and consultant surgeons (CONs). We aim to examine the outcomes of these variables in a local Australian population. We present this article in accordance with the CONSORT reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-2/rc).
Methods
Recruitment and blinding
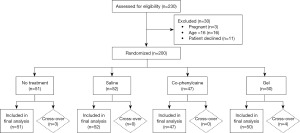
This is a single-centre randomized, double-blind, placebo-controlled, parallel-group study with 1:1:1:1 for four groups. The study was conducted in ENT outpatient clinic at Ipswich Hospital, Queensland, Australia. Allocation of participants was carried out using a computer-generated list of random numbers; participants were stratified via blocked randomization with an allocation of 1:1:1:1 into each trial group. Patients are recruited and randomly varied to achieve a total population of 200 participants (Figure 1). Participants were not privy to block sizes. The two sprays, saline (placebo) and Co-phenylcaine Forte, will be prepared in identical bottles that are multi-use and a new disposable nozzle was used for each patient. Other than taste, the two sprays are prepared to be otherwise identical. One of the research co-ordinators who was not involved in the enrolment, allocation, or intervention prepared the spray bottles.
The study was double-blinded, apart from the use of lubricant, or when no-treatment was administered, which was evident to the examiner. The allocation sequence was concealed from the examiners in sequentially numbered, opaque and sealed envelopes. Patients were not privy to the method of topicalization of any other patient as the procedure was performed in separate rooms, thus keeping allocation blinded. FNE was performed in the outpatient setting in the context of the usual work-up and examination of patients. Sub-group analysis was performed for patients in randomized groups whom were unable to tolerate the examination (did not tolerate, DNT); for these patients, co-phenylcaine Forte topicalization was used for local anaesthesia and mucosal decongestion. There were no patients who were unable to tolerate the examination at all, nor was there any loss-to-follow-up.
FNE was performed by examiners of various levels of training including RMOs, PHOs, REGs and CONs. This information was recorded for use in subgroup analysis, to reflect the variability of examiner in real clinical environment. Although PHOs, REGs, and CONS are already proficient in this type of examination, RMOs regularly rotate through the ENT department every 10 weeks. It is routine as part of their rotation to learn to perform FNE and therefore to reflect normal clinical practice in a public hospital, they were included in this study. However, prior to their participation, they were given a half-hour orientation on the use of the equipment and technique in performing the examination and were under the supervision of an independent senior member of the team for all examinations.
Inclusion/exclusion criteria
Patients included in the trial were over the age of 16 years, and undergoing FNE as part of their routine clinical assessment in the outpatient clinic where they were invited to participate in the study. This included patients who have had previous FNE, which was also recorded for posterity. Any patient with a known allergy to the study medications was excluded. Pregnant or breast-feeding patients were also excluded.
Sample size and statistical analysis
Sample size was determined using a power calculation based on previous literature to achieve a power of 90%. A power calculation using the equation: standard difference = difference between means/population standard deviation; and a 95% confidence interval (CI). See Table 1 for power and sample size calculations for the study outcomes of interest based on available statistics in the published literature.
Table 1
| Outcome measure | Difference in mean | Standard deviation | Standardised difference | Approximate number of patients | Including attrition (30%) |
|---|---|---|---|---|---|
| Discomfort cophenylcaine vs. placebo (4) | |||||
| VAS score | 18.3 | 25.8 | 0.71 | 54 | 71 |
| Pain cophenylcaine vs. placebo (4) | |||||
| VAS score | 11 | 23.4 | 0.47 | 105 | 137 |
| Pain (3) | |||||
| VAS score | 5† | 6 | 0.8 | 42 | 55 |
| Discomfort (3) | |||||
| VAS score | 3.5† | 5.5 | 0.63 | 74 | 97 |
| Unpleasantness (3) | |||||
| VAS score | 3.5† | 5 | 0.7 | 54 | 71 |
| Willingness to repeat (3) | |||||
| VAS score | 1.5† | 4 | 0.4 | 170 | 221 |
| Ease of examination (3) | |||||
| VAS score | 15.5† | 10.5 | 1.48 | 14 | 19 |
| Quality of view (3) | |||||
| VAS score | 2† | 4 | 0.5 | 105 | 137 |
†, using median values. VAS, visual analogue scale.
A Kruskal-Wallis test was used to establish relationships between randomized groups and individual components of the examiner experience. Odds ratios (ORs) were used to determine the outcomes of each pre-treatment group. Statistical significance was defined as P<0.05. Subgroup analysis was performed for the examiner’s level of training/experience level in order to determine if this variable affected patient or examiner outcomes.
VAS questionnaires were conducted by both patient and examiner, and an example of each questionnaire can be found in Appendix 1. Questions were scores from 0–100 pertaining to both the patient experience as well as the examiner experience. Lower VAS scores indicated a more favourable/positive outcome than higher VAS scores. De-identified results were collated, and recorded in an encrypted cloud domain accessible only to an independent research coordinator. Primary outcomes were patient and examiner experience for each method of topicalization. Secondary outcome was investigating the influence of examiner’s level of experience on the results obtained for both primary outcomes.
Ethical clearance
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). IRB approval was obtained prior to data collection (Human Research Ethics Committee, West Moreton Hospital and Health Service, Approval number: HREC|2019|QWMS|57255 [Nov ver 3]). Written informed consent to participate in the study was obtained from the patient prior to commencement of the study.
Results
A total of 200 patients meeting inclusion criteria were successfully recruited for this study between the period of December 2019 to January 2022. The recruited patients included 121 males (60.5%) and 79 females (39.5%), with a median age of 57.38 years (range, 16–89 years). Fifty-one patients were randomized to the “no-treatment” arm, 50 patients were randomized to the “gel” arm, 47 patients were randomized to the “co-phenylcaine” arm, and 52 patients were randomized to the “saline” arm. Recruitment and randomization is shown in Figure 1. Patient demographics within each treatment arm is shown in Table 2. There was no loss-to-follow-up, and no patients were excluded post-recruitment. We present the results of the examiners’ experience and the patients’ e xperiences as follows. Raw data can be found at https://cdn.amegroups.cn/static/public/ajo-23-2-1.xlsx.
Table 2
| Groups | Gender | No. | % within randomised group | Age, years | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | ||||
| Gel | Male | 29 | 58 | 57.38 | 20.96 | 16 | 89 |
| Female | 21 | 42 | 54.52 | 16.15 | 20 | 80 | |
| Co-phenylcaine | Male | 26 | 55.32 | 49.85 | 22.1 | 19 | 84 |
| Female | 21 | 44.68 | 52.52 | 12.46 | 26 | 70 | |
| No treatment | Male | 35 | 68.63 | 56.34 | 19.64 | 21 | 83 |
| Female | 16 | 31.37 | 54.88 | 17.87 | 29 | 76 | |
| Saline | Male | 31 | 59.62 | 53.45 | 16.93 | 21 | 81 |
| Female | 21 | 40.38 | 57.1 | 11.46 | 28 | 75 | |
SD, standard deviation.
Examiner experience/outcomes
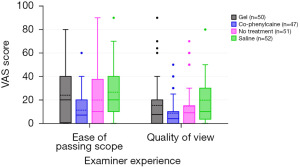
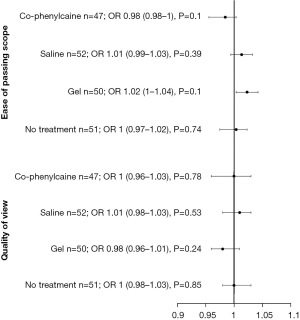
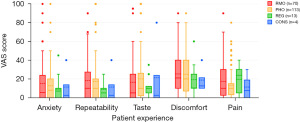
A Kruskal-Wallis test showed that there is a significant difference between the categories of the independent variable RANDOMISED GROUP with respect to the dependent variable QUALITY OF VIEW (Chi2 =8.81, df =3, P=0.03). Similarly, Kruskal-Wallis test showed a significant difference between the categories of the independent variable RANDOMISED GROUP with respect to the dependent variable EASE OF PASSING SCOPE (Chi2 =11.56, df =3, P=0.009). Co-phenylcaine Forte provided the best quality of view, as well as ease of passing scope for the examiner. Examiner experience/outcomes are demonstrated by both VAS scores and as forest plot (Figure 2 and Figure 3 respectively).
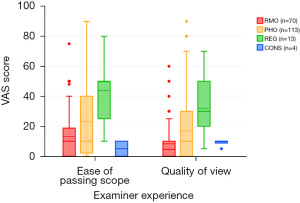
When scrutinizing level of experience and examiner outcomes, a significantly statistically significant association was seen demonstrating lower VAS scores for consultants and resident medical officers (Figure 4), in comparison with PHOs or registrars for both quality of view (Chi2 =21.3, df =3, P<0.001) and ease of passing scope (Chi2 =20.17, df =3, P<0.001).
Patient experience/outcomes
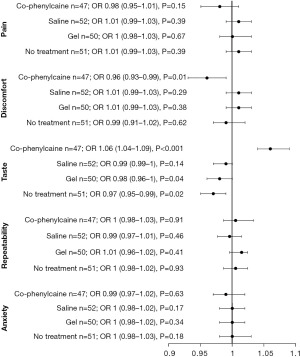
Patient experience for each treatment arm is shown via the reported VAS scores (Figure 5). Pertaining to the patient-reported outcomes, “no-treatment” yielded a statistically significant improvement in overall taste profile (OR, 0.97; 95% CI: 0.95–0.99; P=0.02). Similarly, patients randomized to the “gel” treatment arm reported favourable taste profile (OR, 0.98; 95% CI: 0.96–1.0; P=0.04).
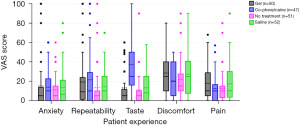
“Co-phenylcaine” treatment yielded lower discomfort scores by patients (OR, 0.96; 95% CI: 0.93–0.99; P=0.01), however it was associated with a significantly poorer taste profile (OR, 1.06; 95% CI: 1.04–1.09; P<0.001). This is best demonstrated in Figure 6.
There was no statistically significant effect of examiner experience on patient-reported outcomes (Figure 7).
DNT cohort
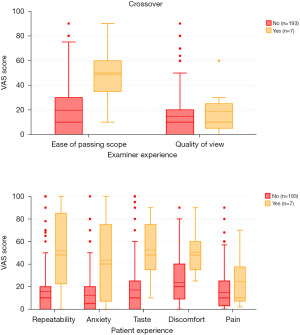
Seven patients required cross-over to Co-phenylcaine Forte spray; four of the patients were originally randomized to the “gel” group, and three of the patients were originally randomized to the “no treatment” group. Median age of DNT patients was 51 years. Three of these patients required cross-over to Co-phenylcaine Forte due to uncontrollable sneezing upon instrumentation, three patients had significantly congested sinonasal mucosa precluding adequate visualization, and one patient required cross-over due to intense anxiety.
With regard to examiner experience in the DNT cohort, while there was no significant correlation between quality of view and need for cross-over (rpb =0.04, n=200, P=0.59), ease of passing scope was significantly associated (rpb =0.24, n=200, P=0.001).
Patient experience was predictably distributed among the DNT cohort. Discomfort (rpb =0.24, n=200, P=0.001), taste (rpb =0.27, n=200, P<0.001), and anxiety (rpb =0.3, n=200, P<0.001) were reportedly all significantly higher in the cross-over group.
Discussion
Our results do not definitively establish and optimum preparation for patients is when performing FNE examination in the outpatient setting. There appear to be advantages and disadvantages to all modalities investigated. The examiner’s level of experience, as one would naturally assume, plays a role in the outcome of the examination. CONs appear to get better views and ease of passing the scope, likely due to heuristics and experience. The apparent paradox that RMOs fare better than expected may be due to relative lack of experience in understanding what an easy scope insertion is or what an optimal endoscopic view looks like.
Best examiner experience
There is limited literature investigating types of topical preparation for nasendoscopy and its effect on the examiner’s experience. A systematic review of the literature was conducted by Conlin & McLean in 2008, in which eight RCTs were included, all using VAS to quantify patients’ experiences of either pain, discomfort, or unpleasantness (5). Only two studies measured endoscopists’ outcomes with incongruent results; one study found that Co-phenylcaine Forte improved the view, however was non-superior to placebo; the other study reported a worse view when using a lubricating agent (5). Pothier et al. demonstrated that gel-lubricant was superior to no-treatment with regard to manoeuvrability of the scope, however subsequent research has demonstrated that water is superior to both no-treatment and gel-lubricant (6,7).
In contrast to the above published results, our study demonstrated that Co-phenylcaine Forte yielded the lowest (most favourable) VAS scores for both “quality of view” and “ease of passing scope”. The combined local anaesthetic and decongestive effects of the spray are the clear explanation for this in physiologically optimising the sinonasal cavity for instrumentation. Gel and saline spray both appeared to hamper one’s quality of view, in comparison with those whom received no treatment. Regarding the performance of lubricant jelly and non-medicated spray, the results of this study appear to differ from those published by Pother et al. It is thought that the “anti-fog” properties from water-spray postulated by Pother et al. (7) may not extend to the use of saline spray as used in this paper.
Best patient experience
There have been several studies conducted investigating topicalization methods to optimize a patient’s experience with nasendoscopy.
In one study which compared lignocaine and phenylephrine, lignocaine alone and xylometazoline with no preparation, more than 80% of patients from each group still experienced some degree of unpleasantness. They found that using a vasoconstrictor alone, which is significantly less expensive, was just as effective as using the combined therapy and that pain was not significantly increased in the absence of local anaesthetic. General unpleasantness was significantly reduced by the vasoconstrictor, but not by the local anaesthetic—this is likely thought to be due to the taste (8). Frosh et al. in 1998 found that the use of xylocaine spray makes the experience worse for the patient, in comparison with no spray, and hypothesized that the psychological effect of the spray caused anticipation of the examination, and therefore a worse overall experience (9). Alternatively, they theorized that the anaesthetic agent could be causing a paradoxical hyperaesthesia to the mucosal lining (9). While not investigated in this study, FNE in the paediatric population has only shown benefit from decongestant use, without the addition of local anaesthetic for topicalization. Median pain and anxiety scores were unaffected (10,11).
This study demonstrates that not providing any form of nasal preparation appears to generally be superior to the other modalities from a patient-perspective. Overall lower VAS scores were recorded for pain, discomfort, taste and repeatability for the patient in the no-treatment arm of the study, in comparison with those whom received with saline spray, gel, or Co-phenylcaine Forte. Anxiety levels were predictably similar across all arms, and largely appears to be independent of what preparation of employed. These findings support the prior research by Frosh et al. with pre-examination topicalization leading to an overall worse patient experience (9). Similarly, we support the hypothesis that topicalization plays a negative psychological role in the patient’s experience during examination.
Co-phenylcaine Forte also yielded low VAS scores for pain and discomfort, as well as repeatability. However, it is of note that taste is a significant issue with Co-phenylcaine Forte.
DNT patients
Sub-group analysis was performed specifically to determine whether there were factors other than local anaesthesia/decongestion that contributed to poor examination tolerance or examiner view. Discomfort, and high anxiety levels were all significant factors which contribute to the examiner needing to cross-over to using Co-phenylcaine Forte preparation (Figure 8). Based on examiner findings, contributing factors to needing to cross-over were predominantly related to unfavourable anatomy (septal spurs, turbinate hypertrophy). Based on this, it appears prudent for an examiner to choose their modality of preparation after some form of anterior rhinoscopy to first determine a baseline understanding for the patient’s sinonasal anatomy prior to instrumentation.
On balance, with Co-phenylcaine Forte being superior than other modalities from an examiner perspective, it appears that individualised preparation may be the gold standard for clinical practice, through a quick discussion between examiner and patient about previous experiences and preference. This will also need to incorporate the examiner’s prior examination of the patient’s nose to determine if there may be any anatomical variations, which should be factored into the decision. This may then need to be tailored to the operators needs to perform an adequate examination, with minimal discomfort to patients. In the absence of any unfavourable sinonasal anatomy, or significantly rhinitic mucosa, no-preparation may be a viable form of preparation for patients undergoing FNE.
Limitations
This study design was partially limited in the blinding capacity of the investigators, primarily with patients randomized to the “gel” and “no-treatment” arms of the study. It could be argued that this could present a degree of observer bias in the examination of these patients, however the results borne of this study would suggest that this effect is negligible. The DNT cohort is also of a small sample size, and results may need to be further validated with a larger study population.
For the purposes of this study, Co-phenylcaine Forte was the only medicated preparation used as the current gold-standard for comparison. It is of note that multiple other decongestants such as oxymetazoline have been proven to be effective for pre-medication of patients (12). Further studies comparing different decongestant preparations may be of value, particularly with regard to general unpleasantness owing to taste which is commonly reported by patients.
It is seen in this study that patients can have a positive experience with no topicalization whatsoever, but at the expense of poorer views for the examiner. Ultimately, it is the authors’ opinion that the best practice would be an individualized approach to each patient, and discussion regarding the different preparation options available, to provide an optimal experience for both patient and examiner.
This trial was not registered, but despite the absence of registration, the trial adheres to the CONSORT statement in order to minimize publication bias and other related issues. We include our trial protocol at https://cdn.amegroups.cn/static/public/ajo-23-2-2.pdf to publish alongside the manuscript in support of this.
Conclusions
There remains a high index of controversy as to what nasal preparation is best when performing a FNE in the outpatient setting. From our study, there does not appear to be any statistically significant difference between any of the treatment groups as it pertains to the examiner experience with quality of view or ease of passing the scope. From the patients’ perspective however, Co-phenylcaine Forte does appear to decrease the discomfort experienced during the examination, however the trade-off for this is an unfavourable taste profile.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-2/rc
Trial Protocol: Available at https://www.theajo.com/article/view/10.21037/ajo-23-2/tp
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-2/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-2/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). IRB approval was obtained prior to data collection (Human Research Ethics Committee, West Moreton Hospital and Health Service, Approval number: HREC|2019|QWMS|57255 [Nov ver 3]). Written informed consent to participate in the study was obtained from the patient prior to commencement of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cain AJ, Murray DP, McClymont LG. The use of topical nasal anaesthesia before flexible nasendoscopy: a double-blind, randomized controlled trial comparing cophenylcaine with placebo. Clin Otolaryngol Allied Sci 2002;27:485-8. [Crossref] [PubMed]
- Georgalas C, Sandhu G, Frosh A, et al. Cophenylcaine spray vs. placebo in flexible nasendoscopy: a prospective double-blind randomised controlled trial. Int J Clin Pract 2005;59:130-3. [Crossref] [PubMed]
- Javed F, Parmar A, Hussain A, et al. A randomised controlled trial assessing the efficacy of co-phenylcaine nasal spray in flexible transnasal pharyngolaryngoscopy. Ann R Coll Surg Engl 2017;99:313-8. [Crossref] [PubMed]
- Bonaparte JP, Javidnia H, Kilty S. A double-blind randomised controlled trial assessing the efficacy of topical lidocaine in extended flexible endoscopic nasal examinations. Clin Otolaryngol 2011;36:550-7. [Crossref] [PubMed]
- Conlin AE, McLean L. Systematic review and meta-analysis assessing the effectiveness of local anesthetic, vasoconstrictive, and lubricating agents in flexible fibre-optic nasolaryngoscopy. J Otolaryngol Head Neck Surg 2008;37:240-9. [PubMed]
- Pothier DD, Awad Z, Whitehouse M, et al. The use of lubrication in flexible fibreoptic nasendoscopy: a randomized controlled trial. Clin Otolaryngol 2005;30:353-6. [Crossref] [PubMed]
- Pothier DD, Raghava N, Monteiro P, et al. A randomized controlled trial: is water better than a standard lubricant in nasendoscopy? Clin Otolaryngol 2006;31:134-7. [Crossref] [PubMed]
- Sadek SA, De R, Scott A, et al. The efficacy of topical anaesthesia in flexible nasendoscopy: a double-blind randomised controlled trial. Clin Otolaryngol Allied Sci 2001;26:25-8. [Crossref] [PubMed]
- Frosh AC, Jayaraj S, Porter G, et al. Is local anaesthesia actually beneficial in flexible fibreoptic nasendoscopy? Clin Otolaryngol Allied Sci 1998;23:259-62. [Crossref] [PubMed]
- Chadha NK, Lam GO, Ludemann JP, et al. Intranasal topical local anesthetic and decongestant for flexible nasendoscopy in children: a randomized, double-blind, placebo-controlled trial. JAMA Otolaryngol Head Neck Surg 2013;139:1301-5. [Crossref] [PubMed]
- Jonas NE, Visser MF, Oomen A, et al. Is topical local anaesthesia necessary when performing paediatric flexible nasendoscopy? A double-blind randomized controlled trial. Int J Pediatr Otorhinolaryngol 2007;71:1687-92. [Crossref] [PubMed]
- Şahin Mİ, Kökoğlu K, Güleç Ş, et al. Premedication Methods in Nasal Endoscopy: A Prospective, Randomized, Double-Blind Study. Clin Exp Otorhinolaryngol 2017;10:158-63. [Crossref] [PubMed]
Cite this article as: Khatri H, Bradshaw K, Kelly S. A randomised placebo-controlled trial of topicalization for flexible nasal endoscopy. Aust J Otolaryngol 2023;6:24.