Acquired subglottic cysts: a case-control study
Introduction
Subglottic cysts (SGCs) are rare but important causes of stridor in neonates. They cause significant upper airway obstruction with potentially lethal consequences, particularly when exacerbated by upper respiratory tract infections. Although laryngeal cysts can be congenital or acquired, most SGCs follow a period of intubation (1,2). Furthermore, there has been an increase in the incidence of acquired SGCs, which is associated with the increased survival of previously intubated, low birth weight, and premature infants (3). A study by Desanto et al. in 1970 showed only one SGC in their case series of 238 laryngeal cysts (4). A study by Bauman and Benjamin in 1995 found that 78% of their patients with laryngeal cysts had SGCs (5).
SGCs are mostly an acquired pathology with formation thought to result from mucosal tissue damage and obstruction of mucous glands. Congenital SGCs are extremely rare (6). Symptoms can range from mild cough to hoarseness, stridor, and respiratory distress.
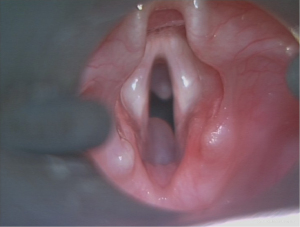
Although a close relationship with endotracheal intubation has been recognised, there has been no definite association between the period of intubation and the risk of developing such a pathology (7). The proposed pathological process is that local trauma from intubation leads to mucosal ulceration, necrosis, fibrosis, and obstruction of the submucosal glands (3). Although well-documented in preterm infants, the predisposing factors leading to the development of these cysts are not readily apparent, and the literature considering these is sparse. Consequently, this study aimed to better understand the circumstances leading to SCGs in the paediatric population (Figure 1). We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-36/rc).
Methods
Study design
We performed a retrospective case-control analysis of patients diagnosed with SGCs at a paediatric tertiary care hospital. Cases were identified by reviewing the operative reports of patients who underwent microlaryngoscopy and bronchoscopy (MLB) at Princess Margaret Hospital for Children (now Perth Children’s Hospital) from January 2009 to January 2014. All patients with SGC who underwent MLB were included in the study. Patients with congenital SGCs or other types of laryngeal cysts were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval (Registration 6173) was obtained from the Child and Adolescent Health Service-Surgical Services GEKOQI Group-Princess Margaret Hospital. Individual consent for this retrospective analysis was waived.
Quantitative variable
Using the birth registry, controls were identified by matching the gestational age at birth and gestational weight with that of the cases. The control group had other indications for MLB, including ruling out congenital and acquired airway abnormalities. The data collected included birth weight, mode of delivery, Apgar scores, need for neonatal resuscitation, and need for endotracheal intubation during resuscitation. We also recorded the duration of endotracheal intubation, number of re-intubations, reason for re-intubation, signs of trauma during intubation, and frequency of endotracheal tube (ETT) suctioning. The data was collected by one senior otolaryngology registrar. No relevant data was missing from the patient records.
The recorded details included symptoms at presentation, age at presentation, age of the patient at MLB, findings on MLB such as the number and location of cysts, degree of airway obstruction, other coexisting airway abnormalities, management of cysts, and patient outcomes. Each case was matched to a control patient by gestational age at birth.
Surgical technique
MLB was performed under general anaesthesia and the airway was exposed using a paediatric Miller intubating laryngoscope. The zero-degree Hopkins rod telescope was used to systematically examine the supraglottic, glottic, and subglottic larynx in detail while the patient’s respirations remained spontaneous. The location and number of SGCs were confirmed and documented. Myer-Cotton classification was used to grade the level of airway obstruction. All procedures were performed under general anaesthesia by one of three senior otolaryngology consultants. Endoscopic treatment of SGCs was treated with marsupialization using a cold steel technique.
Statistical analysis
The data collected were analysed using GraphPad Prism version 5.0b (GraphPad Software, San Diego, CA, USA). Descriptive demographic and clinical data are presented as the mean ± standard deviation (SD). Where appropriate, the Wilcoxon test was used for comparisons between the cases and controls. Differences were considered statistically significant at P<0.05.
Results
Fifteen patients with SGCs were identified from 1,095 patients who underwent MLB. The main presenting symptoms of these patients were stridor or airway obstruction.
Of the 15 patients with SGCs, 14 were born prematurely, with a mean gestational age of 27.1 weeks (23+6–33+1 weeks). One patient who was born at term (39+5 weeks) was intubated on day 11 due to bronchiolitis. All remaining patients were intubated at or shortly after birth. The mean gestational age of the controls was 27.3 weeks (Table 1). The mean gestational weight for the controls was 1,109.5 grams, compared to 1,158.7 grams for the controls.
Table 1
| Clinical variables | SGCs (n=12) | Controls (n=12) | P value |
|---|---|---|---|
| Gestation (weeks) | 27.1±4.0 | 27.3±3.7 | 0.83 |
| Birth weight (grams) | 1,109.5±727.5 | 1,158.7±654.6 | 0.85 |
| Suction frequency (events/day) | 7.3±2.2 | 3.7±1.5 | 0.002 |
| Duration of intubation (hours) | 211±8.7 | 116±9.5 | 0.84 |
| Number of re-intubations | 1.5±1.4 | 1.8±1.1 | 0.62 |
| ETT size (mm) | 2.9±0.2 | 2.9±0.1 | 0.94 |
Data are presented as mean ± SD. SGC, subglottic cyst; ETT, endotracheal tube; SD, standard deviation.
Of the 15 patients with SGCs, 11 presented with stridor, two had recurrent bronchiolitis, and two had symptoms of obstructive sleep apnoea and were found to have SGCs when MLB was performed before adenoidectomy. The age range at presentation was 1 month to 5 years.
Seven patients had bilateral SGCs, and four had SGCs in the posterior subglottis; three were on the left, and two were on the right. Seven patients had >50% of airway obstruction (Cotton-Myer grade 2 subglottic stenosis). Secondary airway abnormalities were commonly noted. Five patients had subglottic stenosis, three had tracheomalacia, and two had tracheobronchomalacia. Fourteen patients underwent endoscopic treatment for SGCs, and one patient with minimal airway obstruction was managed conservatively. Seven of the 14 had recurrent cysts and required revision surgery.
Suction frequency
The frequency of ETT suctioning was calculated as the total number of suctioning events performed during the entire intubation period divided by the number of days that the patients remained intubated. Patients with SGCs had a significantly higher number of ETT suctioning events, with a mean of 7.3 per day, compared to controls, with a mean of 3.7 per day (P=0.002).
Duration of intubation
There was no significant difference in the duration of endotracheal intubation between cases and controls. The length of intubation of the SGC cases varied from 15 h to 475 h and 57 min (mean, 211 h). For the control patients, the duration of intubation ranged from 36 h and 19 min to 889 h and 5 min (mean, 116 h) (P=0.84).
Number of re-intubations
The number of reintubations varied between 0 and 4 for both cases and controls. The average number of reintubations for the SGC group was 1.5, while the control group had an average of 1.8 reintubations (P=0.62).
Size of the ETT
The size of the ETT used and the depth of ETT insertion were selected and determined based on the birth weight of the patients, according to standard clinical protocols. Therefore, no significant difference between the cases and controls was identified, with the average size of the ETT for the SGC group being 2.9 mm and for the control group being 2.9 mm (P=0.94).
There were no differences between the groups in terms of documented pneumonia, sepsis, or pneumothorax.
Discussion
Wigger and Tang first described SGCs in 1968 as a rare cause of subglottic obstruction (3). The increasing survival rates of preterm infants, improved anaesthetic techniques, and superior diagnostic equipment have contributed to a greater awareness and consequent diagnosis of SGCs. Even the most recent publications on SGCs (a systematic review) have failed to identify any obvious risk factors (8).
The pathophysiology of this condition is poorly understood, and there is little literature on risk factors for the development of SGCs. Johnson et al. suggested an increased production and viscosity of mucus in preterm infants and an increased density of the submucous glands in the upper respiratory tract (3). Additionally, they proposed that trauma from endotracheal intubation in the narrower paediatric airway could lead to cyst formation. While these hypotheses allude to both aetiology and risk factors, they fail to explain why most intubated preterm infants do not develop SGCs. Furthermore, it remains unclear what predisposes the preterm infant’s airway to develop SGCs and why they are comparatively almost unheard of in adults. Possibly, SGCs are not large enough to impact the larger calibre adult airway to cause respiratory symptoms or they may spontaneously resolve. A similar but related pathology, namely subglottic stenosis, is associated with preterm birth, especially gestational age <28 weeks, relatively large ETT size (Sherman ratio >0.1), traumatic intubation, multiple intubations, and sepsis (9,10).
One study in 1993 examined 174 preterm infants (mean gestation, 27 weeks) after a minimum of 7 days of intubation. Bronchoscopy was performed in all patients. SGCs were identified in 7% of asymptomatic patients and 10% of patients with airway symptoms (11). Most other studies investigating SGCs have reported a series of cases (ranging from 8 to 55 patients in each study); the pooled mean gestational age was 27.5 weeks, and intubation duration was 18.6 days (11). None of these studies compared the differences between intubated patients who developed SGCs and a control population.
Our study is unique because it compared patients with acquired SGCs to a matched cohort of intubated patients. The mean durations of intubation were 8.8 and 4.8 days, respectively. The gestational age was 27 weeks in both groups. No difference in the period of intubation or gestational age was noted between cases and controls. However, the most significant finding in our study was the difference in the frequency of endotracheal suctioning between the two groups, with the SGC group having more than twice the number of suction events than the control group. Frequent suctioning is usually due to increased secretions observed in conditions such as infection or gastroesophageal reflux disease (GORD). Steehler reported a series of seven cases of SGC that were diagnosed and treated for GORD (the method of diagnosis was not discussed); five of these cases had lipid-laden macrophages on bronchoalveolar lavage, suggestive of aspiration (12).
On histological examination, the subglottic region in premature infants has a greater quantity of submucosal glands, demonstrating increased production of more viscous mucus (7). SGCs are cysts of varying sizes lined with non-ciliated cuboidal and columnar epithelia surrounded by fibroid tissue in the subglottic region (3,13). Together with mucosal trauma caused by ETTs, manipulation of the airway during suctioning and healing by fibrosis may lead to obstruction of the subepithelial glands and ducts as well as formation of these mucosal retention cysts.
Several studies have demonstrated a sequence of acute ulceration, mucosal necrosis, granulation tissue production, and healing with epithelial regrowth, subepithelial fibrosis, and squamous metaplasia (12,14-16). Subepithelial fibrosis and squamous metaplasia can obstruct the ducts of mucous glands in the subglottic region, leading to the formation of retention cysts.
We hypothesised that minor yet frequent manipulations of the ETT may cause sufficient repetitive mucosal trauma. Additionally, the narrow calibre of the paediatric airway may be the reason for minor manipulations causing more significant consequences in the subglottis. Infection or GORD leading to secretion and increased suction requirements may also independently contribute to the development of cysts by causing inflammation or affecting healing.
The time course for developing such cysts varied widely, with the earliest age at first presentation being 1 month. However, at presentation, five of the 15 patients were >12 months, with the oldest being 5 years. Interestingly, the later presentations did not include stridor as the sole presenting symptom. One patient was investigated for obstructive sleep apnoea, but another patient presented with recurrent bronchitis or croup. Furthermore, 11 patients with SGC were noted to have significant upper airway pathology, most often subglottic stenosis or tracheomalacia. The SGC in the 5-year-old patient was considered “small” and identified incidentally during adenoidectomy. Consequently, most symptomatic SGCs were diagnosed at ≤20 months, suggesting that a 2-year follow-up regime would be sufficient to identify most cases.
This study was limited by the relatively small sample size, and the fact that it was a single-centre study.
It is crucial to identify the population prone to developing SGCs, as they may cause airway obstruction, leading to potentially life-threatening events. A higher index of suspicion is needed in infants with increased suction requirements, which may be associated with infection or GORD.
Conclusions
There is an association between SGCs and preterm birth and intubation. Frequent endotracheal suctioning may be associated with the development of SGCs. The damaging effects of repeated or multiple manipulations of the airway, possibly associated with GORD or infection, may put infants at a higher risk of developing SGCs. Consequently, these infants should be closely monitored.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-22-36/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-22-36/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-22-36/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-36/coif). SV serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained from Child and Adolescent Health Service-Surgical Services GEKOQI Group-Princess Margaret Hospital (Registration 6173). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Last SP, Patterson HI, Hauser N, et al. Congenital subglottic cyst: a rare cause for sudden airway compromise in a newborn. BMJ Case Rep 2023;16:e253750. [Crossref] [PubMed]
- Agada FO, Bell J, Knight L. Subglottic cysts in children: a 10-year review. Int J Pediatr Otorhinolaryngol 2006;70:1485-8. [Crossref] [PubMed]
- Johnson LB, Rutter MJ, Shott SR, et al. Acquired subglottic cysts in preterm infants. J Otolaryngol 2005;34:75-8. [Crossref] [PubMed]
- DeSanto LW, Devine KD, Weiland LH. Cysts of the larynx--classification. Laryngoscope 1970;80:145-76. [Crossref] [PubMed]
- Bauman NM, Benjamin B. Subglottic ductal cysts in the preterm infant: association with laryngeal intubation trauma. Ann Otol Rhinol Laryngol 1995;104:963-8. [Crossref] [PubMed]
- Smith SP, Berkowitz RG, Phelan PD. Acquired subglottic cysts in infancy. Arch Otolaryngol Head Neck Surg 1994;120:921-4. [Crossref] [PubMed]
- Wigger HJ, Tang P. Fatal laryngeal obstruction by iatrogenic subglottic cyst. J Pediatr 1968;72:815-20. [Crossref] [PubMed]
- Soloperto D, Spinnato F, Di Gioia S, et al. Acquired subglottic cysts in children: A rare and challenging clinical entity. A systematic review. Int J Pediatr Otorhinolaryngol 2021;140:110523. [Crossref] [PubMed]
- Thomas RE, Rao SC, Minutillo C, et al. Severe acquired subglottic stenosis in neonatal intensive care graduates: a case-control study. Arch Dis Child Fetal Neonatal Ed 2018;103:F349-54. [Crossref] [PubMed]
- Suzumura H, Nitta A, Tanaka G, et al. Role of infection in the development of acquired subglottic stenosis in neonates with prolonged intubation. Pediatr Int 2000;42:508-13. [Crossref] [PubMed]
- Downing GJ, Hayen LK, Kilbride HW. Acquired subglottic cysts in the low-birth-weight infant. Characteristics, treatment, and outcome. Am J Dis Child 1993;147:971-4. [Crossref] [PubMed]
- Steehler MK, Groblewski JC, Milmoe GJ, et al. Management of subglottic cysts with Mitomycin-C-A case series and literature review. Int J Pediatr Otorhinolaryngol 2011;75:360-3. [Crossref] [PubMed]
- Halimi C, Nevoux J, Denoyelle F, et al. Acquired subglottic cysts: management and long term outcome. Int J Pediatr Otorhinolaryngol 2012;76:589-92. [Crossref] [PubMed]
- Lim J, Hellier W, Harcourt J, et al. Subglottic cysts: the Great Ormond Street experience. Int J Pediatr Otorhinolaryngol 2003;67:461-5. [Crossref] [PubMed]
- Gould SJ, Young M. Subglottic ulceration and healing following endotracheal intubation in the neonate: a morphometric study. Ann Otol Rhinol Laryngol 1992;101:815-20. [Crossref] [PubMed]
- Joshi VV, Mandavia SG, Stern L, et al. Acute lesions induced by endotracheal intubation. Occurrence in the upper respiratory tract of newborn infants with respiratory distress syndrome. Am J Dis Child 1972;124:646-9. [Crossref] [PubMed]
Cite this article as: Last S, Ng CH, Wood J, Rao S, Vijayasekaran S. Acquired subglottic cysts: a case-control study. Aust J Otolaryngol 2023;6:23.