
A comparison between tropical and temperate climatic conditions on epistaxis presentation rates in Australia
Introduction
Spontaneous epistaxis is a common otorhinolaryngology condition and accounts for many patient presentations to emergency departments (EDs) throughout Australia. Historically the lifetime incidence of epistaxis has been reported up to 60% (1,2), yet robust epidemiological data regarding the condition is lacking due to a high degree of non-presenter bias as most episodes are self-limiting. In the United States of America, it accounts for up to 33% of emergent admissions with respect to ear, nose, and throat pathology (3) and to date there is a paucity of published data regarding hospital presentations in Australia for the condition.
Classical teaching proposes the frequency of epistaxis tends to be higher in cooler seasons when ambient air temperature and relative humidity are lower than in warmer months. Previous studies at single institutions outside Australia have supported seasonal variation with respect to epistaxis when analysing extremes of temperature occurring at higher latitudes and have demonstrated negative correlation between frequency of epistaxis and rising temperature and variable correlation with respect to humidity (4-9). There are no studies to date that directly compare presentation rates in patient populations from markedly differing climatic conditions. Australia has significant variation in climate ranging from humid sub-tropical and tropical conditions in the north, arid and dry conditions in western regions and cool temperate conditions in Southern districts.
This multi-center epidemiological study sought to compare presentation rates for spontaneous epistaxis at Cairns Hospital in tropical North Queensland to Austin Hospital in temperate Victoria and correlate these with climatic variables from the Australian Bureau of Meteorology (BOM) to better understand trends in Australian epistaxis rates. We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-10/rc).
Methods
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol and methods were reviewed and approved by the Health and Medical Human Research Ethics Committee at the Austin Hospital (Reference: Audit/19/Austin/73) and Cairns Hospital (Reference: LNR/2019/QCH/55438). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Patient data
A retrospective analysis of patients presenting to EDs with idiopathic spontaneous epistaxis from July 2014 through December 2018 at both Cairns Hospital in Queensland and Austin Hospital in Melbourne, Victoria was conducted. Potential confounding variables including nasal trauma and recent nasal surgery were excluded from the dataset such that only idiopathic epistaxis was included for analysis. Furthermore, the total number of daily patient presentations to the ED of each hospital was recorded and used as a denominator for calculation of daily epistaxis ratios which represent epistaxis presentations as a percentage of total ED presentations.
Patient demographics including age, sex and length of stay were collected. Patient clinical history including anticoagulant/antiplatelet status and the co-existence of medical co-morbidities including hypertension, the presence of hematological disorders/malignancy, chronic kidney disease, hepatic dysfunction and obstructive sleep apnoea were also recorded. Where clinical characteristics were not available or reported in the medical record it was assumed the characteristic was not present.
Meteorological data
Historical weather variables recorded by the Australian BOM were extracted from the National Library of Australia online archives (10). Minimum and maximum daily values were averaged to estimate mean daily temperature (°C) and mean daily relative humidity (%) over the study period. Mean daily dew point values are not captured by the BOM and were instead calculated using the National Oceanic and Atmospheric Administration formula (11). These weather variables were then correlated with the occurrence of epistaxis on a monthly and seasonal basis. The seasons were divided as follows: winter was defined as June to August, spring was defined as September to November, summer was defined as December to February, and autumn was defined as March to May based on Australian meteorological system.
Statistical analysis
In this study, descriptive analysis and statistical inference were carried out to investigate relationships of variables. Descriptive statistics were used to describe demographics and clinical characteristics of epistaxis cases. Chi-square tests with two-way contingency tables were used to analyse categorical variables. Numerical data was analysed using one-way analysis of variance (ANOVA) test to report prevalence of epistaxis presentations. We analysed seasonal and monthly frequency of epistaxis incidence with meteorological variables using Pearson’s correlation analysis. Correlation coefficients (r) between −0.4 and +0.4 were considered as weak correlations, between +0.4 and +0.7 or −0.4 and −0.7 as moderate correlations, and ≥0.7 or ≤−0.7 as strong correlations (12). A value near ‘zero’ means that there is a random, non-linear association between variables. Univariate linear regression analysis between the number of epistaxis cases per month and mean monthly temperature (°C), mean monthly humidity (%) and mean monthly dew point were performed. A 2-tailed value of P<0.05 was considered statistically significant.
Results
A total of 1,622 cases of epistaxis were identified after exclusions with 1,260 and 362 episodes recorded at Austin Hospital and Cairns Hospital respectively (Figure 1). Patient demographics and clinical characteristics (Table 1) demonstrated a higher average age of presentation at Austin compared to Cairns with a similar gender bias towards male presenters however this bias was not statistically significant at either institution.
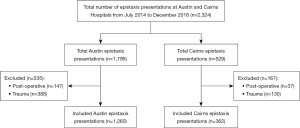
Table 1
| Clinical characteristics | Cairns (n=362) | Austin (n=1,260) |
|---|---|---|
| Age (years) | 49.64±27.1 | 66.08±22.4 |
| Sex | ||
| Male | 214 (59.1) | 742 (58.9) |
| Female | 148 (40.9) | 518 (41.1) |
| Anti-platelet/anti-coagulants | ||
| Yes | 153 (42.3) | 860 (68.3) |
| No | 209 (57.7) | 400 (31.7) |
| Hypertension | 171 (47.2) | 657 (52.1) |
| Pro-thrombotic (haematologic disorder/malignancy) | 17 (4.7) | 28 (2.2) |
| Chronic kidney disease | 10 (2.8) | 95 (7.5) |
| Liver disease | 9 (2.5) | 40 (3.2) |
| Obstructive sleep apnoea | 5 (1.4) | 43 (3.4) |
| Length of stay (days) | 0.67±1.4 | 0.63±1.1 |
Data are presented as mean ± standard deviation or n (%).
Average monthly temperature and humidity was consistently higher in Cairns compared with Austin while average monthly dew point was higher in the winter and early spring months at Austin and lower in the warmer months compared with Cairns (Table 2). Neither temperature, humidity nor dew point varied significantly across the four and a half year reporting period (July 14th to December 18th).
Table 2
| Season | Month | Austin | Cairns | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean monthly temperature (°C) | Mean monthly humidity (%) | Mean monthly dew point (°C) | Epistaxis ratio by month | Epistaxis ratio by season | Mean monthly temperature (°C) | Mean monthly humidity (%) | Mean monthly dew point (°C) | Epistaxis ratio by month | Epistaxis ratio by season | |||
| Summer | January | 21.5 | 59.5 | 12.8 | 0.27 | 0.26 | 28.3 | 69.8 | 22.2 | 0.11 | 0.08 | |
| February | 21.1 | 59.2 | 12.6 | 0.22 | 28.0 | 72.3 | 22.5 | 0.08 | ||||
| Autumn | March | 20.1 | 60.1 | 11.6 | 0.30 | 0.32 | 28.0 | 72.7 | 22.4 | 0.09 | 0.11 | |
| April | 16.7 | 64.5 | 9.5 | 0.32 | 26.4 | 68.4 | 19.8 | 0.10 | ||||
| May | 14.1 | 66.5 | 7.7 | 0.32 | 25.0 | 68.3 | 18.5 | 0.13 | ||||
| Winter | June | 11.0 | 75.3 | 6.6 | 0.40 | 0.40 | 23.2 | 69.3 | 17.0 | 0.15 | 0.14 | |
| July | 11.0 | 68.0 | 5.2 | 0.39 | 22.2 | 65.1 | 15.2 | 0.12 | ||||
| August | 11.4 | 66.1 | 5.1 | 0.43 | 22.4 | 59.8 | 14.0 | 0.16 | ||||
| Spring | September | 13.4 | 60.5 | 5.6 | 0.37 | 0.32 | 24.0 | 58.9 | 15.3 | 0.14 | 0.14 | |
| October | 16.3 | 57.2 | 7.5 | 0.32 | 25.3 | 60.7 | 17.0 | 0.15 | ||||
| November | 17.9 | 59.2 | 9.3 | 0.28 | 27.2 | 60.7 | 18.9 | 0.12 | ||||
| Summer | December | 20.3 | 58.2 | 11.4 | 0.28 | 0.26 | 28.0 | 64.6 | 20.6 | 0.06 | 0.08 | |
Epistaxis ratios for each institution demonstrated a consistently and significantly higher rate of presentation at Austin compared to Cairns across months (Table 2). Furthermore, ANOVA showed statistically significant differences across months for Austin (P=0.02) but not Cairns (P=0.17). When pooled for seasonality however (Table 2), ANOVA demonstrated significance for both Austin (P<0.001) and Cairns (P=0.01).
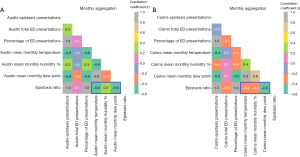
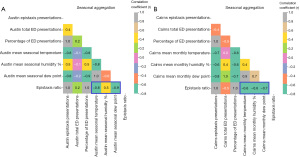
Pearson correlation analysis demonstrated negative correlation between epistaxis ratio and mean monthly temperature at Austin [r=−0.6, P<0.001, 95% confidence interval (CI): −0.73, −0.36] and Cairns (r=−0.4, P=0.004, 95% CI: −0.59, −0.12) as well as negative correlation between epistaxis ratio and mean monthly dew point at both Austin (r=−0.6, P<0.001, 95% CI: −0.71, −0.33) and Cairns (r=−0.5, P<0.001, 95% CI: −0.65, −0.23) (Figure 2). When pooled for seasonality (Figure 3), higher negative correlation was demonstrated between epistaxis ratio and temperature at Austin (r=−0.8, P<0.001, 95% CI: −0.93, −0.63) and Cairns (r=−0.6, P=0.006, 95% CI: −0.83, −0.20) as well as dew point at Austin (r=−0.9, P<0.001, 95% CI: −0.94, −0.64) and Cairns (r=−0.7, P<0.001, 95% CI: −0.89, −0.42). At Austin there was mild positive correlation between epistaxis ratio and mean monthly humidity (r=0.3, P=0.01, 95% CI: 0.06, 0.54) and mean monthly seasonal humidity (r=0.5, P=0.01, 95% CI: 0.12, 0.80). At Cairns this correlation was negative for both mean monthly humidity (r=−0.4, P=0.003, 95% CI: −0.59, −0.13) and mean seasonal humidity (r=−0.6, P=0.004, 95% CI: −0.84, −0.33).
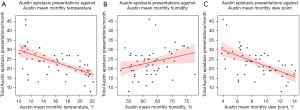
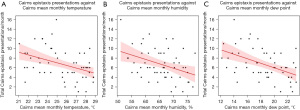
Univariate linear regression analysis demonstrated moderate and statistically significant negative relationships between total epistaxis presentations per month and mean monthly temperature (r=−0.58, P<0.001) and dew point (r=−0.57, P<0.001) for Austin Hospital (Figure 4). Moderate and statistically significant negative relationships were also demonstrated for Cairns Hospital (Figure 5) when comparing epistaxis presentations and mean monthly temperature (r=−0.40, P=0.002) and dew point (r=−0.47, P<0.001).
Discussion
This study sought to investigate the relationship between epistaxis presentation rates and climatic variables in two Australian locations with distinctly differing climates. Evidence of seasonal fluctuations and epistaxis remains quite heterogenous in the literature. The National Hospital Ambulatory Medical Care Survey in the United States demonstrated higher rates of epistaxis in the winter months of December through February (13). Sowerby et al. (4) were able to demonstrate significant negative correlation between epistaxis rates and temperature in Alberta, Canada that is classified as a continental climate where mean daily temperatures are often significantly below 0 °C in the winter months. More recently, McMillin et al. (14) investigated climatic and epistaxis correlations in Saint John, New Brunswick in Canada that experiences a maritime climate with less extreme seasonal variation compared with Alberta. They also found higher rates of epistaxis in winter as well as negative correlation between daily humidity and epistaxis. In contrast Yu et al. (15) looked at 6,805 cases of paediatric epistaxis in subtropical Zhejiang on the east coast of China and found strong positive correlation between epistaxis incidence and temperature.
This study supports previous findings of a statistically significant negative correlation between epistaxis presentations and ambient air temperature in both temperate and tropical climates. We found this correlation was most significant at Austin which, compared to Cairns, experiences lower average temperatures and more defined seasonality. We demonstrated moderate negative correlation between epistaxis incidence and humidity in Cairns however this was not replicated at Austin which showed weak positive correlation suggesting the combination of humidity and warm air in Cairns provides some protective effect with respect to epistaxis incidence.
It is postulated the combination of low ambient temperature, indoor heating and reduced relative humidity results in increased water evaporation from the respiratory tract and thus increased intranasal mucosal desiccation (16). Few studies have investigated the relationship between dew point and epistaxis presentation rates (15). Dew point represents the temperature which air must be cooled to become saturated in water vapour and has a significant relationship to human thermoregulation. Australians in subtropical/tropical climates typically experience discomfort with rising dew point because of reduced evaporative cooling, however discomfort also exists when dew point is very low as drier air leads to dry skin and dry mucous membranes in the respiratory tract. We found there was a significant negative correlation between dew point and epistaxis rates for both Austin and Cairns and this was highest at Austin (r=−0.9) when adjusted for seasonality. As dew point depends on both temperature and relative humidity for its measurement, we postulate it may be a more important factor for epistaxis than humidity alone. As such, traditional preventative strategies such as the utilisation of home warm air humidification systems in winter for patients prone to epistaxis would seem well supported by this finding.
Interestingly epistaxis presentations as a percentage of total ED presentations were consistently at least twice as high for Austin Hospital compared to Cairns across all time periods. We noted heterogeneity between the groups with respect to average patient age with a significantly higher proportion of elderly patients presenting to Austin Hospital (Table 1). Previous epidemiological analyses of epistaxis have consistently demonstrated a bimodal age distribution with higher rates of presentations in young children and elderly patients (17). We referenced the Australian Bureau of Statistics (18) and compared the median age from population data for local government areas around Austin (median age 37.2 years) and Cairns Hospitals (median age 39.0 years) and did not find any significant population bias to explain these results. Patients taking either or a combination of anti-platelet and anti-coagulation medications was also significantly higher for Austin patients which would be expected given the age distribution being skewed towards elderly presenters and this could potentially be a confounding factor. Sample sizes for patient clinical characteristics were not sufficient large enough to conduct subgroup analysis, we propose however the higher and more consistent ambient temperature, humidity and dewpoints of the Cairns tropical climate may have some role in explaining the lower overall epistaxis ratio and may play a role with respect to the lower mean age of total presentations.
This study was limited by the retrospective design and that we did not control for clinical co-morbidities except for known causes of epistaxis such as trauma and recent nasal surgery. Non-presenter bias is another limiting factor in determining the true effect of climate on epistaxis as the majority of spontaneous idiopathic nose bleeds resolve with simple first aid and do not require presentation to hospital; this is a limitation of all studies on this topic to date. Although we have compared patients from two unique climates, both institutions are relatively coastal from a geographic point of view and future inclusion of a hospital situated in an inland hot dessert climate may add further insight into the variable effects of climate on epistaxis.
Conclusions
This is the first Australian study to characterise climatic predictors of epistaxis and the first study to directly compare patients from markedly differing climates. We found strong and statistically significant negative correlation between temperature, as well as dew point and epistaxis incidence which was highest when adjusted for seasonality. Furthermore, we demonstrated a consistently higher burden of epistaxis across all time periods in an ED situated in a temperate climate compared to one in a tropical climate despite similar population demographics. This study supports previous conclusions on the variable effect of climate on epistaxis and lends credence to traditional preventative strategies in managing this problematic condition.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-10/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-10/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-10/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol and methods were reviewed and approved by the Health and Medical Human Research Ethics Committee at the Austin Hospital (Reference: Audit/19/Austin/73) and Cairns Hospital (Reference: LNR/2019/QCH/55438). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petruson B, Rudin R. The frequency of epistaxis in a male population sample. Rhinology 1975;13:129-33. [PubMed]
- Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg 2007;15:180-3. [Crossref] [PubMed]
- Morgan DJ, Kellerman R. Epistaxis: evaluation and treatment. Prim Care 2014;41:63-73. [Crossref] [PubMed]
- Sowerby LJ, DeSerres JJ, Rudmik L, et al. Role of season, temperature and humidity on the incidence of epistaxis in Alberta, Canada. J Otolaryngol Head Neck Surg 2014;43:10. [Crossref] [PubMed]
- Bray D, Giddings CE, Monnery P, et al. Epistaxis: are temperature and seasonal variations true factors in incidence? J Laryngol Otol 2005;119:724-6. [Crossref] [PubMed]
- Comelli I, Vincenti V, Benatti M, et al. Influence of air temperature variations on incidence of epistaxis. Am J Rhinol Allergy 2015;29:e175-81. [Crossref] [PubMed]
- Min SJ, Kang H, Kim KS, et al. Minimal temperature, mean wind speed, and mean relative humidity are associated with spontaneous epistaxis in Seoul, Korea. Auris Nasus Larynx 2021;48:98-103. [Crossref] [PubMed]
- Matsumoto S, Ishii R, Kiuchi C, et al. Effect of Average Relative Humidity on Epistaxis. Cureus 2023;15:e36063. [Crossref] [PubMed]
- Danielides V, Kontogiannis N, Bartzokas A, et al. The influence of meteorological factors on the frequency of epistaxis. Clin Otolaryngol Allied Sci 2002;27:84-8. [Crossref] [PubMed]
- Daily weather observations, National Library of Australia. [accessed Sep 16, 2019]. Available online: https://webarchive.nla.gov.au/tep/44065
- Physical Sciences Labratory, National Oceanic and Atmospheric Administration, 2021. [accessed Nov 17, 2019]. Available online: https://psl.noaa.gov/data/composites/day/calculation.html
- Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012;24:69-71. [PubMed]
- Pallin DJ, Chng YM, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med 2005;46:77-81. [Crossref] [PubMed]
- McMullin B, Atkinson P, Larivée N, et al. Examining seasonal variation in epistaxis in a maritime climate. J Otolaryngol Head Neck Surg 2019;48:74. [Crossref] [PubMed]
- Yu G, Fu Y, Dong C, et al. Is the occurrence of pediatric epistaxis related to climatic variables? Int J Pediatr Otorhinolaryngol 2018;113:182-7. [Crossref] [PubMed]
- Chaaban MR, Zhang D, Resto V, et al. Demographic, Seasonal, and Geographic Differences in Emergency Department Visits for Epistaxis. Otolaryngol Head Neck Surg 2017;156:81-6. [Crossref] [PubMed]
- Mangussi-Gomes J, Enout MJ, Castro TC, et al. Is the occurrence of spontaneous epistaxis related to climatic variables? A retrospective clinical, epidemiological and meteorological study. Acta Otolaryngol 2016;136:1184-9. [Crossref] [PubMed]
- Data by region, Australian Bureau of Statistics, 2021. [accessed Feb 02, 2023]. Available online: https://dbr.abs.gov.au/
Cite this article as: Lomas J, Yii N, Tescher A, Mansouri N, Vo H, Aung AK, Teh BM. A comparison between tropical and temperate climatic conditions on epistaxis presentation rates in Australia. Aust J Otolaryngol 2023;6:22.


