
Surgically managed symptomatic ecchordosis physaliphora: a systematic review
Introduction
Ecchordosis physaliphora (EP) first described in the literature by Luschka in 1856, is a benign, congenital gelatinous lesion arising from vestigial notochordal remnants (1,2). EPs are typically found along the midline craniospinal axis, with the most common location being at the clivus or the prepontine surface (1,3). Here, it is often asymptomatic and diagnosed on autopsies, with the incidence reported to be between 0.5% to 5% (4).
Conversely chordomas, which are also notochordal in origin and represent up to 4% of primary bone tumours, are considered the malignant counterpart of EPs and its main differential diagnosis (5). It characteristically occurs within the extradural intraosseous region, and due to its tendency to exhibit aggressive features such as local bony infiltration and osteolytic destruction, chordomas often manifest with neurological symptoms and signs including cranial nerve palsies (4-6).
EP and chordoma remain a diagnostic challenge due to its common embryonic origins and thus almost identical histopathological characteristics. Consequentially, considering the challenging nature of distinguishing between the two notochordal derived lesions, a multimodal approach to determining a diagnosis is required as the prognostication and management implications differ significantly. Despite the traditionally asymptomatic nature of EPs which can be monitored with serial imaging, there have been numerous cases of symptomatic presentations requiring operative management reported in the literature (Table 1). This differs from chordomas which are reported to have a 45.2% 3-year recurrence-free survival rate, whereby a more aggressive approach including gross total resection and potentially adjuvant radiotherapy is required (6).
Table 1
| Author | Year | Age (years) | Sex | Symptoms | Duration (month) | CSF leak | Meningitis | Location | MRI | Surgical approach | Immunohistochemistry | Follow-up† (month) |
Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | Contrast-enhancement | |||||||||||||
| MacDonald (1) | 1990 | 66 | F | CSF rhinorrhoea, anosmia | 24 | Yes | No | Prepontine | ↑ | ↑ | Non-enhancing | Sublabial midline rhinoseptal transsphenoidal approach | Cytokeratin+, S100+ | 4 | No recurrence |
| Watanabe (7) | 1994 | 48 | M | Right hearing disturbance, facial paraesthesia | – | No | No | Clival | ↓ | ↑ | Heterogenous enhancement | Right suboccipital craniotomy | EMA+, cytokeratin+, S100+ | 10 | Mild right facial paresis |
| Akimoto (8) | 1996 | 51 | F | Headache, transient diplopia | – | No | No | Prepontine | – | – | Non-enhancing | Right retrolabyrinthine pre-sigmoid | Low MIB-1 | – | – |
| Toda (9) | 1998 | 56 | F | Persistent headache | 3 | No | No | Prepontine | ↓ | ↑ | Non-enhancing | Left lateral suboccipital craniotomy | Vimentin+, EMA+, S100+, Ki-67 antigen−, proliferating cell nuclear antigen− | 24 | No recurrence |
| Rodríguez (10) | 1999 | 54 | F | Dizziness | 18 | – | – | Prepontine | ↓ | ↑ | Non-enhancing | Right suboccipital craniotomy | Vimentin+, EMA+, cytokeratin+, S100+, glial fibrillary acidic protein+, CD-68−, low MIB-1 | – | – |
| Cha (11) | 2002 | 49 | M | Dizziness, headache, gait instability | 1 | No | No | Prepontine | ↓ | – | – | Transmaxillary Lefort 1 transclival approach | EMA+, cytokeratin+, S100+, low MIB-1 | 18 | No recurrence |
| Takeyama (5) | 2006 | 12 | M | Left hemiparesis, diplopia | – | No | No | Prepontine | ↓ | ↑ | Non-enhancing | Subtotal resection | EMA+, cytokeratin+, S100+, glial fibrillary acidic protein−, low MIB-1 | 6 | No recurrence |
| Ling (12) | 2007 | 45 | M | Acute left hearing loss, non-pulsatile tinnitus | – | No | No | Prepontine | ↓ | ↑ | Non-enhancing | Transpetrosal approach to the CPA | Cytokeratin+, S100+, low MIB-1 | 1 | No recurrence |
| Rotondo (13) | 2007 | 47 | M | Headache, right facial pain | 3 | No | No | Prepontine | ↓ | ↑ | Non-enhancing | Pre-sigmoidal approach with complete mastoidectomy | – | 12 | No recurrence |
| Miki (14) | 2008 | 59 | M | Gait disturbance, dizziness | 1 | No | No | Prepontine | ↓ | ↑ | – | Endoscopic trans-third ventricle approach | – | 36 | No recurrence |
| Alli (15) | 2008 | 52 | F | Clear left rhinorrhoea | 2 | Yes | Yes | Prepontine | ↓ | ↑ | Non-enhancing | EEA → complicated by further leak → transsphenoidal repair of clival defect | – | 24 | No recurrence |
| Yamamoto (16) | 2013 | 20 | F | Diplopia, CN VI palsy | Acute | No | No | Retroclival prepontine | ↑ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) subtotal resection | Cytokeratin+, Ki-67−, low MIB-1 | 12 | No recurrence |
| Krisht (17) | 2013 | 16 | F | Headaches, diplopia, CN VI palsy | 2 | No | No | Extra-axial epidural | ↔ | ↑ | Non-enhancing | Microscopic to ventral clival wall subtotal resection | Low MIB-1 | 30 | Complete and persistent resolution of diplopia. Stable residual lesion |
| Dias (18) | 2014 | 54 | F | Fever, headaches, meningism, clear rhinorrhoea | 6 | Yes | Yes | Prepontine | ↓ | ↑ | Non-enhancing | EEA | EMA+, cytokeratin+, S100− | 24 | No recurrence |
| Choudhri (19) | 2014 | 63 | M | Progressive bilateral tremor of upper limbs, intermittent headaches | – | No | No | Prepontine | ↓ | ↑ | Non-enhancing | EEA | Low Ki-67 | 21 | No residual tumour. Headaches and tremor resolved 15 months post-operatively |
| Bolzoni-Villaret (patient 1) (20) |
2014 | 51 | F | Clear rhinorrhoea | 36 | No | No | Clival | – | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | – | 12 | No recurrence |
| Bolzoni-Villaret (patient 2) (20) |
2014 | 39 | F | Left CN VI palsy, left retro-orbital pain, diplopia since age 17 | 264 | No | No | Prepontine | ↓ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | – | 12 | No recurrence |
| Ferguson (21) | 2016 | – | F | Headache, confusion | Acute | Yes | Yes | Prepontine | ↓ | ↑ | Non-enhancing | EEA | Cytokeratin+, S100+, low Ki-67 | – | – |
| Zhong (22) | 2015 | 34 | M | Left diplopia | 12 | No | No | Prepontine | ↓ | ↑ | Non-enhancing | Left frontotemporal craniotomy subtotal resection | Low MIB-1 | – | Stable residual tumour |
| Filis (23) | 2016 | 44 | F | Occipital headache, neck pain, nausea | 6 | No | No | Prepontine | ↓ | ↑ | Non-enhancing | Left frontotemporal craniotomy | EMA+, S100+, glial fibrillary acidic protein−, low Ki-67 | 12 | Facial weakness and slurred speech post-operatively. Resolved at 1 year follow-up. No recurrence |
| Adib (24) | 2016 | 57 | M | Diplopia, right CN VI palsy, left body paraesthesia | 24 | No | No | Prepontine | ↓ | ↑ | Non-enhancing | Endoscopic trans-third ventricular procedure | Cytokeratin+, S100− | 6 | Diplopia and paraesthesia resolved. Six-month MRI: near total excision of EP |
| T1 | T2 | Contrast-enhancement | |||||||||||||
| Miki (25) | 2017 | 44 | F | Right facial dysesthesia | 120 | No | No | Prepontine to inter-peduncular | ↓ | ↑ | Non-enhancing | Anterior transpetrosal approach | EMA+, cytokeratin+, S100+, low MIB-1 | 5 | Symptoms improved |
| Lindemann (26) | 2019 | 35 | F | Headache | – | No | No | Clival | ↓ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | Cytokeratin+, S100+ | 10 | No recurrence |
| Galloway (27) | 2019 | 40 | F | Acute meningitis, clear rhinorrhoea | 1 | Yes | Yes | Clival | – | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | EMA+, cytokeratin+, S100+ | 36 | No recurrence |
| Georgalas (patient 1) (28) | 2020 | 81 | M | Acute bacterial meningitis, clear rhinorrhoea | Acute | Yes | Yes | Prepontine | – | ↑ | – | EEA (transsphenoidal transclival) | Vimentin+, EMA+, cytokeratin+, S100+ | 28 | Symptom free |
| Georgalas (patient 2) (28) | 2020 | 60 | M | Clear rhinorrhoea, meningitis | 0.5 | Yes | Yes | Prepontine | ↓ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | Vimentin+, EMA+, cytokeratin+, S100+ | 20 | Symptom free |
| Georgalas (patient 3) (28) | 2020 | 64 | F | Meningitis, clear rhinorrhoea | 72 | Yes | Yes | Prepontine | ↓ | ↑ | – | EEA (transsphenoidal transclival) | Vimentin+, EMA+, cytokeratin+, S100+ | 7 | Symptom free |
| Sun (29) | 2020 | 22 | F | Severe headaches, horizontal diplopia | 12 | No | No | Retroclival | ↓ | ↑ | Non-enhancing | EEA | Cytokeratin+, S100+ | 8 | No recurrence |
| Ghimire (30) | 2020 | 65 | F | Reduced consciousness, progressive mutism, right hemiparesis | Acute | No | No | Prepontine | – | ↑ | – | EEA | EMA+, cytokeratin+, S100+, low Ki-67 | 0.5 | CSF rhinorrhoea requiring re-exploration and repair‡ |
| Ang (31) | 2020 | 43 | F | Bitemporal and occipital headache | 120 | No | No | Retroclival prepontine | ↓ | ↑ | Non-enhancing | Transsphenoidal clival biopsy | EMA+, cytokeratin+, S100+ | – | CSF leak requiring 2 repairs. Asymptomatic on next neurosurgical follow-up |
| Derakhshani (32) | 2020 | 68 | F | Worst headache, imbalance, clear rhinorrhoea | Acute | Yes | No | Retroclival | – | ↑ | Non-enhancing | EEA | Cytokeratin+, low Ki-67 | 2 | Occasional and trace sensation of rhinorrhoea |
| Veiceschi (patient 1) (33) | 2021 | 59 | M | CSF leak, meningitis | – | Yes | Yes | Prepontine | ↓ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | Cytokeratin+, S100+, low Ki-67 | 36 | No recurrence |
| Veiceschi (patient 2) (33) | 2021 | 64 | F | CSF leak | – | Yes | No | Prepontine | ↓ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | EMA+, cytokeratin+, low Ki-67, low MIB-1 | 24 | No recurrence |
| Veiceschi (patient 3) (33) | 2021 | 41 | M | CSF leak post head trauma | – | Yes | No | Prepontine | ↓ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | EMA+, cytokeratin+, S100+, low Ki-67, low MIB-1 | 72 | No recurrence |
| Veiceschi (patient 4) (33) | 2021 | 39 | F | Right CN VI palsy | – | – | – | Prepontine | ↓ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | Cytokeratin+, S100+, low Ki-67, low MIB-1 | 36 | No recurrence |
| Veiceschi (patient 5) (33) | 2021 | 57 | M | CSF leak post head trauma | – | Yes | – | Prepontine | ↓ | ↑ | Non-enhancing | EEA (transsphenoidal transclival) | Cytokeratin+, S100+, low Ki-67 | 18 | No recurrence |
| Sooltangos (patient 1) (34) | 2022 | 65 | F | Acute confusion, left weakness, malaise, anorexia, lethargy | Acute | – | – | Clival | – | ↑ | Non-enhancing | EVD insertion, EEA | EMA+, cytokeratin+, S100+, Ki-67− | 3 | Further endoscopic multilayered repair§. Three-month CT and MRI: no residual or recurrent tumour |
| Sooltangos (patient 2) (34) | 2022 | 39 | F | Headaches, CSF rhinorrhoea | 6 | Yes | Yes | Clival | ↓ | ↑ | Non-enhancing | EEA | EMA+, S100+, low Ki-67 | 8 | Post-operative meningitis treated. Follow-up 8-month MRI stable |
| Sooltangos (patient 3) (34) | 2022 | 43 | F | Bacterial meningitis, rhinorrhoea | 0.25 | Yes | Yes | Clival | ↓ | ↑ | Non-enhancing | EEA | EMA+, S100+, low Ki-67 | – | Clinically stable |
| Sooltangos (patient 4) (34) | 2022 | 45 | F | Head injury following collapse, rhinorrhoea | 12 | Yes | No | Clival | ↓ | ↑ | Non-enhancing | EEA | – | – | – |
| Raffa (35) | 2022 | 7 | M | Persistent headache | – | No | No | Prepontine | ↔ | ↑ | Non-enhancing | EEA | Vimentin+, EMA+, cytokeratin+, S100+, low Ki-67 | 12 | No recurrence |
| Hasegawa (36) | 2023 | 41 | F | Headaches, CSF rhinorrhoea | 1 | Yes | No | Clival | ↓ | – | Non-enhancing | EEA | – | – | – |
†, month of last follow-up are reported; ‡, fat graft, repositioning of nasoseptal flap; §, abdominal fat graft, dural substitute, nasoseptal flap. ↑, hyperintense; ↓, hypointense; ↔, isointense. EP, ecchordosis physaliphora; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; F, female; M, male; EMA, epithelial membrane antigen; CPA, cerebellopontine angle; EEA, endoscopic endonasal approach; CN, cranial nerve; EVD, extraventricular drain; CT, computed tomography.
At present, there are several systematic reviews in the literature describing symptomatic EPs, such as Veiceschi et al. and Stuebe et al. (33,37). However, in this paper, our primary objective is to showcase a comprehensive analysis of operative cases of EP, encompassing a thorough evaluation of various crucial aspects. Specifically, we aim to provide valuable insights into patient demographics, clinical presentations, imaging modalities, operative approaches, and outcomes. To enrich the readers’ understanding and enhance the significance of our findings, we have made dedicated efforts to include a more extensive collection of case studies and series, thereby bolstering the robustness of our systematic review. Furthermore, we have incorporated visual aids, such as intra-operative endoscopic images and detailed histopathological findings, to further elucidate the nuances of EP management. By incorporating these diverse elements, we believe our paper will offer a comprehensive and invaluable resource for those seeking to advance their knowledge of EP and its operative management. We present this article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-17/rc).
Methods
Information sources and search strategy
We performed a comprehensive search of PubMed, Ovid MEDLINE, Ovid EMBASE, and Cochrane Database of Systematic Reviews from inception to January 2023. This was carried out in accordance with the PRISMA reporting guidelines, using the terms “ecchordosis physaliphora” with no language restriction (Appendix 1). Further cases were identified through cross-referencing of studies to identify and retrieve articles that were potentially missed during the initial database search process. This study was conducted in accordance with the Declaration (as revised in 2013). Due to the retrospective nature of the research involving published case reports and case series, the requirement for ethics approval was waived, and appropriate ethical considerations have been duly adhered to throughout the entirety of this research endeavour.
Inclusion and exclusion criteria
This systematic review includes case studies, case reports, case series with or without literature reviews. The preliminary screening process was performed based on review of the titles and abstracts. Articles describing symptomatic cases of EP where operative management was performed, with histological confirmation of EP were included. The exclusion criteria consisted of the following: asymptomatic lesions, EP identified on autopsy, cases of EP that were conservatively managed with surveillance or corticosteroids, cases diagnosed based on imaging, EPs situated in locations outside of the clival or prepontine region and histopathological diagnoses of chordomas or intradural chordomas. Full-text articles not written in English were not reviewed unless official translation was available.
Study selection
Titles and abstracts were independently screened by two authors (PY Toh and JL Tan), those that did not fulfil the inclusion criteria were excluded. Full-text articles of the selected studies were subsequently obtained and independently assessed by both authors. Disagreements amongst the two authors were resolved with discussion and consensus between the two authors. Data obtained from each study were individually entered into an Excel spreadsheet.
Data items
These cases were then further evaluated and patient demographics such as age at presentation and gender, presenting symptoms, associated symptoms, presence of meningitis, confirmation of cerebrospinal fluid (CSF) leakage with beta-2 transferrin, tumour location, imaging findings, surgical approach, intra-operative findings, and follow-up were recorded.
Data analysis methods
Quantitative data was analysed using IBM® SPSS v28 (IBM Corporation, Armonk, NY, USA), and qualitative data items were summarised and tabulated provided in the “Results” section.
Results
Results of search strategy
The search criteria resulted in the identification of 296 published studies (Figure 1), with 4 additional records retrieved from cross-referencing. A total of 131 articles were screened for review of eligibility following removal of duplicates. Close review of titles and abstracts identified 85 irrelevant records.
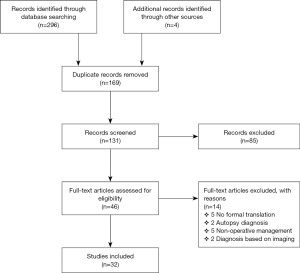
Forty-six full-text articles were screened for eligibility and 14 articles were ultimately omitted based on the inclusion and exclusion criterion. Of the 14, 5 were non-English articles due to the inability to retrieve copies with formal translation. A total of 32 eligible full-text articles were thoroughly analysed for this systematic review. Amongst the 32 studies, there were 42 individual cases of symptomatic EP managed operatively.
Characteristics of included studies
All eligible studies included were either case reports or case series, with 6 articles including a literature review and 7 case reports incorporating surgical techniques (Table 1). One article included a systematic review of literature surrounding EP between the years 1990 and 2020.
Synthesis of results
Sufficient information was available to collate demographic and clinical data for each case, with the mean and percentages calculated and summarised in Table 2. The mean age at presentation is 47.3 years, with most cases presenting between the 4th and 5th decade of life. There was also a preponderance of EP amongst females (64.3%). Clinically, 40.5% of patients had a CSF leak, 38.1% reported headaches, with 23.8% developing or presenting with meningitis. Imaging and histopathological features were also collated to identify common features of EP that contribute to its diagnosis (Table 1). EPs were typically found to be located within the prepontine region, with magnetic resonance imaging (MRI) demonstrating features of T1 hypointensity, T2 hyperintensity, and these lesions were non-gadolinium enhancing.
Table 2
| Parameters | Data |
|---|---|
| Age (years) | |
| Mean [range] | 47.3 [7–81] |
| ≤20 years | 3 (7.3) |
| 21–39 years | 7 (17.0) |
| 40–59 years | 22 (53.7) |
| ≥60 years | 9 (22.0) |
| Gender | |
| Male | 15 (35.7) |
| Female | 27 (64.3) |
| Presenting symptoms | |
| CSF rhinorrhoea | 17 (40.5) |
| Headaches | 16 (38.1) |
| Meningitis | 10 (23.8) |
| Abducens nerve palsy | 5 (11.9) |
| Duration of symptoms (months) | |
| Median [range] | 6 [0.25–264] |
Data are presented as mean [range] or n (%). CSF, cerebrospinal fluid.
Following the year 2008, cases were largely managed with an endoscopic endonasal approach (Table 3) which reflects the increased popularity of endoscopic skull base surgery during this time (38). With regards to cases that did not undergo radical surgery, four subtotal resections were performed, and a transsphenoidal clival biopsy was performed in one case. In the post-operative period, four cases were complicated by a CSF leak requiring return to theatre and repair, with one of the cases requiring two repairs with a multilayered approach. Other complications include one case that developed mild right facial paresis following a right suboccipital craniotomy, one developed facial weakness and slurred speech that resolved at the 1-year mark and one developed post-operative meningitis treated with intravenous antibiotics. The mean follow-up period was 17.3 months with 23 of the 37 radical resection cases demonstrating no recurrence on imaging and 1 was found to have near-total excision of EP. Three patients reported to be symptom-free, 1 had improved symptoms, and 1 was reported to be clinically stable. Eight cases did not provide specific follow-up data on symptomatology, recurrence or residual tumour.
Table 3
| Approaches | N (%) |
|---|---|
| EEA | 26 (61.9) |
| Suboccipital craniotomy | 5 (11.9) |
| Presigmoid | 2 (4.8) |
| Transmaxillary transclival | 1 (2.4) |
| Transpetrosal | 1 (2.4) |
| Endoscopic trans-third ventricle | 2 (4.8) |
| Sublabial midline rhinoseptal transsphenoidal | 1 (2.4) |
| Transpetrosal | 2 (4.8) |
| Microscopic | 2 (4.8) |
EEA, endoscopic endonasal approach.
Study of the risk of bias
In accordance with our selection criteria, the studies encompassed within our analysis were exclusively comprised of case reports and case series. Among these, 1 paper integrated a systematic literature review, while an additional 6 articles concurrently presented literature reviews. Extracting pertinent data from these sources allowed us to discern patient demographics, prevalent clinical manifestations, significant radiological and histopathological characteristics, as well as the outcomes associated with the surgical management of symptomatic EP. Notably, imaging and histopathological assessments were instrumental in elucidating discernible traits during the diagnostic phase of this relatively obscure condition.
Given the observational nature of these studies and the absence of comprehensive operative outcome analyses, a formal evaluation of bias risk was not conducted. Nevertheless, we undertook a quality appraisal utilizing the assessment tool for case reports and case series developed by the Joanna Briggs Institute (JBI) Centre for Evidence-Based Health Care. This evaluation was independently executed by two authors. It is important to acknowledge that potential biases inherent in the included studies might impact our findings and conclusions.
Case report
We present an unpublished case report from our unit as part of this systematic review. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
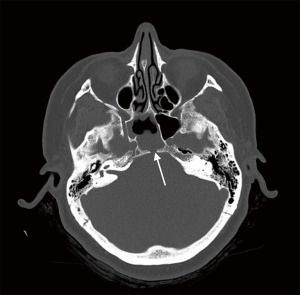
A 51-year-old female presented to her General Practitioner with a 1-week history of persistent clear anterior rhinorrhoea and low-pressure, throbbing headaches with no visual or neurological symptoms. A computed tomography (CT) of her head and sinuses was organized, which demonstrated a defect within the posterior aspect of the sphenoidal sinus with an associated opacification in the sphenoid sinus raising the possibility of a CSF leak (Figure 2). She was subsequently referred to our ear, nose, and throat (ENT) department.
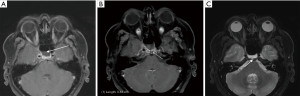
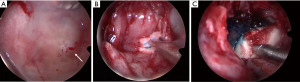
The patient represented to the emergency department (ED) a few days later with severe low-pressure headaches and was admitted for workup of possible meningitis. A lumbar puncture performed was unremarkable. An MRI identified a T1 hypointense, T2 hyperintense, and non-gadolinium enhancing lesion (Figure 3), and the patient subsequently underwent endoscopic repair of CSF leak. An endoscopic endonasal transsphenoidal approach was undertaken, with a bilateral sphenoidotomy which identified a 5 mm bony defect along the middle third of the clivus with exposed dura and a CSF leak. A soft lesion was then found to be adherent to the exposed dura and was subsequently resected and sent for histopathology (Figure 4A). For the multilayered CSF leak repair, the left nasoseptal flap was raised, a fibrin sealant patch TachoSil® (Baxter, Deerfield, IL, USA) was then placed over the bony defect, followed by the nasoseptal flap, dural sealant DuraSeal® (Integra LifeSciences Corporation, Plainsboro, NJ, USA), nasal packing inclusive of a bioresorbable nasal dressing Nasopore® (Stryker Corporation, Kalamazoo, MI, USA), and silastic splints (Figure 4B,4C). She had an uncomplicated recovery and was discharged day 6 post-operatively.
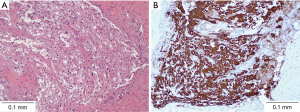
Histopathological analysis demonstrated rounded and uniform nuclei with fine chromatin, moderate volumes of pale, often vacuolated eosinophilic cytoplasm, forming physaliphorous cells (Figure 5). There was no evidence of hypercellularity, atypia, mitotic activity, necrosis or any poorly differentiated components. Immunohistochemistry staining demonstrated diffuse uptake for cytokeratins AE1/AE3, CK8/18, and epithelial membrane antigen (EMA), with patchy staining for S100. MIB-1 was not significantly elevated. A post-operative MRI performed at the 6-month mark showed no significant residual lesion in the prepontine region.
Discussion
EP is a benign, typically asymptomatic hamartoma that arises from embryonic notochordal remnants. It is often described as a small, jelly-like nodule situated in the intradural prepontine mid-clival region, level with Dorello’s canal, typically less than 2 cm in size (39). Embryologically, the notochord is described as a primitive vertebral column that is formed during the third week of gestation before completely regressing within the first year of life, and this structure eventually forms the nucleus pulposus of the intervertebral discs in adulthood (13,40). However, the fate of the notochordal cells is variable at the clival and sacral ends of the notochord due to a convoluted course deterring these cells from their natural path (19,39). This ultimately results in remnant or heterotopic cells, first described by Luschka in 1856 before being described microscopically by Virchow in 1857, which give rise to notochordal tumours in the axial skeleton during adulthood (1,2,19,39,41).
Another tumour arising from notochordal remnants is a chordoma, which accounts for 1–4% of all bone malignancies (42). These lesions contrast significantly in terms of their behaviour relative to EPs as they demonstrate osteolytic destructive activity and arise extradurally in the bone, with a third of them being situated in the clivus (43,44). They are generally insidiously slow growing, spreading over critical bony and neural structures, making definitive treatment challenging (44).
EPs are frequently asymptomatic and found on autopsy examinations. Nevertheless, despite their indolent nature, they can become more apparent following gradual tumour expansion, compression on surrounding structures requiring operative intervention, and in some rare cases result in intratumoural or fatal pontine haemorrhage as evidenced by the literature in Table 1 (45,46). Common presentations described include headaches, CSF rhinorrhoea, meningitis, abducens nerve palsy, dizziness, hearing disturbance or loss, gait instability and facial dysesthesias or paraesthesia (1,7,9,12-14,17,19,20,25). Conversely, due to the locally destructive nature of chordomas, patients typically present symptomatically with severe headaches and cranial nerve palsies, thus often requiring radical surgical debulking and adjuvant radiotherapy.
Within the clivus, aside from chordomas, there are several other differentials to consider, such as chondrosarcomas, fibrous dysplasia, meningiomas, primary lymphomas, leiomyomas in immunocompromised patients, and metastases (40,42,47). Owing to the difference in prognosis and management between EPs, chordomas and the listed differentials, it is pertinent to distinguish the entities, thus predominantly relying on further imaging alongside histopathological and immunohistochemical features to arrive at a diagnosis. For the purpose of the systematic review, focus will be centred on classical imaging and histopathological features of EP and how they differ from that of chordomas. In terms of imaging, CT and MRI are the primary imaging modalities used to facilitate diagnosis. CT features are usually limited in assisting with the diagnosis of EP compared to MRI, as this modality is predominantly used to identify a clival defect, as well as for assessing for an associated osseous or cartilaginous clival stalk (39,48). In MRIs, EPs appear expansive with T1 hypointensity and T2 hyperintensity. Furthermore, these lesions often do not enhance with the addition of gadolinium contrast, differentiating them from chordomas which tend to contrast enhance, likely due to the presence of increased vascularity (7). All three features have been consistently cited in the literature (Table 1) and is felt to be a reliable indicator of an EP (39,41,48). We have however, identified a few cases in our review that did not meet the above radiological features criteria. These included MacDonald and Yamamoto et al.’s cases whereby both patients’ MRIs demonstrated both T1 and T2 hyperintensity and non-enhancement with Gadolinium, with immunohistochemical and histopathological diagnosis of EP (1,16). Additionally, Krisht et al. and Raffa et al.’s patients demonstrated T1 isointensity on MRI instead (17,35). Another important characteristic of chordomas that are distinct from EPs are that the former, despite its frequent tendency to be locally destructive possesses the ability to metastasize in up to 40% of cases, archetypally to loco-regional lymph nodes, liver, lungs, and bone (42,49).
In terms of the histopathological component of diagnosing EPs, this too presents ongoing challenges due to both notochordal tumours exhibiting similar features, such as the presence of physaliphorous cells under electron microscopy, and comparable immunohistochemical patterns. Physaliphora arises from the Greek term ‘physalis’ which means “bubble”, and these cells are typically described as large, intracytoplasmic mucinous vacuoles with intermediate filaments situated within a mucopolysaccharide-rich myxoid matrix (46,50). It is deemed a hallmark feature of notochord-derived lesions. Furthermore, immunohistochemistry findings include positive stains for EMA, vimentin, S100 protein and cytokeratins AE1 and AE3 (43,46,50,51). EPs are found to demonstrate regularity, with the absence of hypercellularity, pleomorphism, atypia or local bony destruction and infiltration unlike chordomas, which are arranged in cords separated by fibrous septa, and also appear to have a greater proliferation rate (34,50,51,52). Thus, a higher MIB-1 (>5%) or Ki-67 index would favour a diagnosis of a chordoma instead of EP, which was consistent with our review of the literature when specific immunostainings were available (25,34,50,51,52). Due to the similitudes, a diagnosis of EP should be made in conjunction with radiological findings. At times, a multidisciplinary approach with an otolaryngology skull base surgeon, neurosurgeon, neuroradiologist and pathologist is favoured.
EPs are frequently incidental findings on imaging, making serial radiological surveillance a reasonable form of management. However, operative management should be considered in symptomatic cases. Based on our literature review, it was evident that almost all operative cases of EP described prior to the year 2008 were performed via non-endoscopic endonasal approaches. These included three cases which underwent suboccipital craniotomies, a sublabial midline rhinoseptal transsphenoidal microscopic approach, a retrolabyrinthine presigmoid approach, a transpetrosal approach to the cerebellopontine angle, and a pre-sigmoidal approach with complete mastoidectomy (1,5,7,8,9,11,12,13,53). The exception during this period was in 2002, when Cha et al. described a transmaxillary (Lefort 1) transclival resection of EP through an upper buccal sulcus incision, followed by the use of 0°, 30°, and 70° rigid endoscopes to inspect the tumour bed and border. Its advantage in enabling the surgeon to obtain a broader view of the central skull base and prepontine region and identify potential sites of residual tumour not entirely visible or exposed with the microscope was acknowledged (11,54). Nonetheless, it was noted that the utilisation of endoscopy in skull base or clival resections were still rare at the time of writing. Following this, Yamamoto et al. described the first successful use of the exclusively endoscopic endonasal transsphenoidal approach in the subtotal resection of a symptomatic EP (16). With the adaptation of this minimally invasive approach, there has not been a recorded recurrence of EP thus far.
Additionally, the endoscopic endonasal approach to repair of CSF leaks has also proven to be effective. Of the 16 cases requiring primary repair of CSF leak, 14 cases described adopting a multilayered approach with varying combinations of a graft (fat, fascia lata, or iliotibial), nasoseptal flap, and dural sealant, fibrin sealant or absorbable haemostats (16,18,19,20,27,28,32,33,34). One case described only using a fat graft during repair, and another case where a biopsy was performed was repaired with a nasoseptal flap alone (21,34). Two cases were unfortunately complicated by further CSF rhinorrhoea requiring a return to theatre. Ghimire et al.’s case underwent a primary nasoseptal flap repair and fibrin sealant which on return to theatre, was re-explored and a fat graft was applied along with repositioning of the formerly harvested nasoseptal flap (30). Sooltangos et al.’s first case of the series which was mended primarily with a nasoseptal flap required further endoscopic repair with a multilayered approach consisting of an abdominal fat graft, a dural substitute, and a nasoseptal flap (34). Overall, the endoscopic endonasal approach to managing symptomatic EPs has proven to be effective with reduced peri-operative morbidity. Nevertheless, it is paramount to consider that in the presence of heavy tumour load, minimally invasive surgery may not be suitable, thus warranting surgeon familiarity with both traditional and novel techniques (19).
Conclusions
Symptomatic EP and its primary differential diagnosis, clival chordomas, pose significant challenges during the diagnostic phase due to their overlapping characteristics. Given the divergent prognoses and subsequent treatment implications, our study emphasizes the distinct radiological and histological attributes of EP, aiming to enhance its identification. This includes highlighting that post-resection management for EP diverges from that of chordomas; while the latter necessitates adjuvant therapy due to the high recurrence risk, EP management predominantly stands independent of adjuvant measures. Additionally, we delve into operative strategies, particularly underscoring the benefits and safety of the endoscopic endonasal approach for symptomatic EP resection.
It is crucial to acknowledge that the evidence underpinning this review has certain limitations, including the reliance on case reports and case series, which inherently offer a lower level of evidence.
In terms of future research directions, a pivotal stride towards translating our findings into clinical utility involves establishing a comprehensive framework for EP surveillance. We propose that forthcoming research should prioritize longitudinally monitoring the disease trajectory by conducting extended follow-ups of operative cases. This endeavour not only allows for a deeper understanding of the natural course of the condition but also lays the groundwork for informed recommendations concerning the optimal duration and intensity of surveillance for patients afflicted with these lesions.
Acknowledgments
The authors would like to thank the Sir Charles Gairdner Hospital Anatomical Pathology department for providing microscopy photographs.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-17/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-17/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-17/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, for the published case reports and case series in the literature review, the requirement for ethics approval was waived, and appropriate ethical considerations have been duly adhered to throughout the entirety of this research endeavour. Written informed consent was obtained from the patient of the unpublished case report for publication of this study and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macdonald RL, Cusimano MD, Deck JH, et al. Cerebrospinal fluid fistula secondary to ecchordosis physaliphora. Neurosurgery 1990;26:515-8; discussion 518-9. [Crossref] [PubMed]
- Sarasa JL, Fortes J. Ecchordosis physaliphora: an immunohistochemical study of two cases. Histopathology 1991;18:273-5. [Crossref] [PubMed]
- Park HH, Lee KS, Ahn SJ, et al. Ecchordosis physaliphora: typical and atypical radiologic features. Neurosurg Rev 2017;40:87-94. [Crossref] [PubMed]
- Sun X, Hornicek F, Schwab JH. Chordoma: an update on the pathophysiology and molecular mechanisms. Curr Rev Musculoskelet Med 2015;8:344-52. [Crossref] [PubMed]
- Takeyama J, Hayashi T, Shirane R. Notochordal remnant-derived mass: ecchordosis physaliphora or chordoma? Pathology 2006;38:599-600. [Crossref] [PubMed]
- Roberti F, Sekhar LN, Jones RV, et al. Intradural cranial chordoma: a rare presentation of an uncommon tumor. Surgical experience and review of the literature. J Neurosurg 2007;106:270-4. [Crossref] [PubMed]
- Watanabe A, Yanagita M, Ishii R, et al. Magnetic resonance imaging of ecchordosis physaliphora--case report. Neurol Med Chir (Tokyo) 1994;34:448-50. [Crossref] [PubMed]
- Akimoto J, Takeda H, Hashimoto T, et al. A surgical case of ecchordosis physaliphora. No Shinkei Geka 1996;24:1021-5. [PubMed]
- Toda H, Kondo A, Iwasaki K. Neuroradiological characteristics of ecchordosis physaliphora. Case report and review of the literature. J Neurosurg 1998;89:830-4. [Crossref] [PubMed]
- Rodríguez L, Colina J, López J, et al. Intradural prepontine growth: giant ecchordosis physaliphora or extraosseous chordoma? Neuropathology 1999;19:336-40. [Crossref]
- Cha ST, Jarrahy R, Yong WH, et al. A rare symptomatic presentation of ecchordosis physaliphora and unique endoscope-assisted surgical management. Minim Invasive Neurosurg 2002;45:36-40. [Crossref] [PubMed]
- Ling SS, Sader C, Robbins P, et al. A case of giant ecchordosis physaliphora: a case report and literature review. Otol Neurotol 2007;28:931-3. [Crossref] [PubMed]
- Rotondo M, Natale M, Mirone G, et al. A rare symptomatic presentation of ecchordosis physaliphora: neuroradiological and surgical management. J Neurol Neurosurg Psychiatry 2007;78:647-9. [Crossref] [PubMed]
- Miki T, Nakajima N, Akimoto J, et al. Neuroendoscopic trans-third ventricle approach for lesions of the ventral brainstem surface. Minim Invasive Neurosurg 2008;51:313-8. [Crossref] [PubMed]
- Alli A, Clark M, Mansell NJ. Cerebrospinal fluid rhinorrhea secondary to ecchordosis physaliphora. Skull Base 2008;18:395-9. [Crossref] [PubMed]
- Yamamoto T, Yano S, Hide T, et al. A case of ecchordosis physaliphora presenting with an abducens nerve palsy: A rare symptomatic case managed with endoscopic endonasal transsphenoidal surgery. Surg Neurol Int 2013;4:13. [Crossref] [PubMed]
- Krisht KM, Palmer CA, Osborn AG, et al. Giant ecchordosis physaliphora in an adolescent girl: case report. J Neurosurg Pediatr 2013;12:328-33. [Crossref] [PubMed]
- Dias LA, Nakanishi M, Mangussi-Gomes J, et al. Successful endoscopic endonasal management of a transclival cerebrospinal fluid fistula secondary to ecchordosis physaliphora--an ectopic remnant of primitive notochord tissue in the clivus. Clin Neurol Neurosurg 2014;117:116-9. [Crossref] [PubMed]
- Choudhri O, Feroze A, Hwang P, et al. Endoscopic resection of a giant intradural retroclival ecchordosis physaliphora: surgical technique and literature review. World Neurosurg 2014;82:912.e21-6. [Crossref] [PubMed]
- Bolzoni-Villaret A, Stefini R, Fontanella M, et al. Transnasal endoscopic resection of symptomatic ecchordosis physaliphora. Laryngoscope 2014;124:1325-8. [Crossref] [PubMed]
- Ferguson C, Clarke DB, Sinha N, et al. A Case Study of Symptomatic Retroclival Ecchordosis Physaliphora: CT and MR Imaging. Can J Neurol Sci 2016;43:210-2. [Crossref] [PubMed]
- Zhong XL, Huang B, Liu C, et al. Multiple Ecchordosis Physaliphora: A Challenging Diagnosis. Chin Med J (Engl) 2015;128:2826-8. [Crossref] [PubMed]
- Filis A, Kalakoti P, Nanda A. Symptomatic ecchordosis physaliphora mimicking as an intracranial arachnoid cyst. J Clin Neurosci 2016;28:171-4. [Crossref] [PubMed]
- Adib SD, Bisdas S, Bornemann A, et al. Neuroendoscopic Trans-Third Ventricular Approach for Surgical Management of Ecchordosis Physaliphora. World Neurosurg 2016;90:701.e1-6. [Crossref] [PubMed]
- Miki K, Yoshimoto K, Nishimura A, et al. A Case of Ecchordosis Physaliphora in the Prepontine Cistern: A Rare Entity in the Differential Diagnosis of an Epidermoid Cyst. World Neurosurg 2017;105:1033.e11-4. [Crossref] [PubMed]
- Lindemann TL, Kamrava B, Chakraborty B. Endoscopic Transsphenoidal Management of Ecchordosis Physaliphora in the Sphenoid Sinus: A Case Report and Review of Approaches to Resection. J Head Neck Surg 2019;2:18-23.
- Galloway L, Hayhurst C. Spontaneous cerebrospinal fluid rhinorrhoea with meningitis secondary to ecchordosis physaliphora. Br J Neurosurg 2019;33:99-100. [Crossref] [PubMed]
- Georgalas C, Terzakis D, Tsikna M, et al. Ecchordosis physaliphora: a cautionary tale. J Laryngol Otol 2020;134:46-51. [Crossref] [PubMed]
- Sun R, Ajam Y, Campbell G, et al. A Rare Case of Ecchordosis Physaliphora Presenting With Headache, Abducens Nerve Palsy, and Intracranial Hypertension. Cureus 2020;12:e8843. [Crossref] [PubMed]
- Ghimire P, Shapey J, Bodi I, et al. Spontaneous tension pneumocephalus and pneumoventricle in ecchordosis physaliphora: case report of a rare presentation and review of the literature. Br J Neurosurg 2020;34:537-42. [Crossref] [PubMed]
- Ang LN, Kew TY, Toh CJ, et al. Ecchordosis physaliphora masquerading as chordoma: a case report. Hong Kong J Radiol 2020;23:223-6. [Crossref]
- Derakhshani A, Livingston S, William C, et al. Spontaneous, Intrasphenoidal Rupture of Ecchordosis Physaliphora with Pneumocephalus Captured During Serial Imaging and Clinical Follow-Up: Pathoanatomic Features and Management. World Neurosurg 2020;141:85-90. [Crossref] [PubMed]
- Veiceschi P, Arosio AD, Agosti E, et al. Symptomatic ecchordosis physaliphora of the upper clivus: an exceedingly rare entity. Acta Neurochir (Wien) 2021;163:2475-86. [Crossref] [PubMed]
- Sooltangos A, Bodi I, Ghimire P, et al. Do All Notochordal Lesions Require Proton Beam Radiotherapy? A Proposed Reclassification of Ecchordosis Physaliphora as Benign Notochord Cell Tumor. J Neurol Surg B Skull Base 2022;83:e96-e104. [Crossref] [PubMed]
- Raffa A. Atypical Presentation and Neuroradiological Features of Giant Ecchordosis Physalyphora in a Seven-Year-Old Patient: A Case Report. Cureus 2022;14:e23544. [Crossref] [PubMed]
- Hasegawa H, Van Gompel JJ, Choby G, et al. Unrecognized notochordal lesions as a likely cause of idiopathic clival cerebrospinal fluid leaks. Clin Neurol Neurosurg 2023;224:107562. [Crossref] [PubMed]
- Stuebe CM, Rindler RS, Laack N, et al. Evaluation of Long-Term Follow-Up in Ecchordosis Physaliphora versus Chordoma. World Neurosurg 2023;174:157-68. [Crossref] [PubMed]
- Doglietto F, Prevedello DM, Jane JA Jr, et al. Brief history of endoscopic transsphenoidal surgery--from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus 2005;19:E3. [Crossref] [PubMed]
- Nardi C, Maraghelli D, Pietragalla M, et al. A practical overview of CT and MRI features of developmental, inflammatory, and neoplastic lesions of the sphenoid body and clivus. Neuroradiology 2022;64:1483-509. [Crossref] [PubMed]
- Rai R, Iwanaga J, Shokouhi G, et al. A comprehensive review of the clivus: anatomy, embryology, variants, pathology, and surgical approaches. Childs Nerv Syst 2018;34:1451-8. [Crossref] [PubMed]
- Lagman C, Varshneya K, Sarmiento JM, et al. Proposed Diagnostic Criteria, Classification Schema, and Review of Literature of Notochord-Derived Ecchordosis Physaliphora. Cureus 2016;8:e547. [Crossref] [PubMed]
- Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. Lancet Oncol 2012;13:e69-76. [Crossref] [PubMed]
- Yuh SJ, Woulfe J, Corsten MJ, et al. Diagnostic imaging dilemma of a clival lesion and its clinical management implications. J Neurol Surg B Skull Base 2014;75:177-82. [Crossref] [PubMed]
- Ciarpaglini R, Pasquini E, Mazzatenta D, et al. Intradural clival chordoma and ecchordosis physaliphora: a challenging differential diagnosis: case report. Neurosurgery 2009;64:E387-8; discussion E388. [Crossref] [PubMed]
- Stam FC, Kamphorst W. Ecchordosis physaliphora as a cause of fatal pontine hemorrhage. Eur Neurol 1982;21:90-3. [Crossref] [PubMed]
- Adamek D, Malec M, Grabska N, et al. Ecchordosis physaliphora - a case report and a review of notochord-derived lesions. Neurol Neurochir Pol 2011;45:169-73. [Crossref] [PubMed]
- Kunicki J, Rzewuska N, Kunicki M, et al. A Rare Case of a Primary Leiomyoma of the Clivus in an Immunocompetent Patient and a Review of the Literature Regarding Clival Lesions. Diagnostics (Basel) 2022;13:9. [Crossref] [PubMed]
- Huang JH, Hagiwara M. Skull Base Tumor Mimics. Neuroimaging Clin N Am 2022;32:327-44. [Crossref] [PubMed]
- Chen G, Li M, Xu W, et al. Surgical Outcomes of Clival Chordoma Through Endoscopic Endonasal Approach: A Single-Center Experience. Front Endocrinol (Lausanne) 2022;13:800923. [Crossref] [PubMed]
- Macdonald RL, Deck JH. Immunohistochemistry of ecchordosis physaliphora and chordoma. Can J Neurol Sci 1990;17:420-3. [Crossref] [PubMed]
- Salisbury JR, Isaacson PG. Demonstration of cytokeratins and an epithelial membrane antigen in chordomas and human fetal notochord. Am J Surg Pathol 1985;9:791-7. [Crossref] [PubMed]
- Amer HZ, Hameed M. Intraosseous benign notochordal cell tumor. Arch Pathol Lab Med 2010;134:283-8. [Crossref] [PubMed]
- Wolfe JT 3rd, Scheithauer BW. "Intradural chordoma" or "giant ecchordosis physaliphora"? Report of two cases. Clin Neuropathol 1987;6:98-103. [PubMed]
- Crockard HA. Transclival surgery. Br J Neurosurg 1991;5:237-40. [Crossref] [PubMed]
Cite this article as: Toh PY, Ling S, Wong D, Tan JL. Surgically managed symptomatic ecchordosis physaliphora: a systematic review. Aust J Otolaryngol 2023;6:20.


