
Clinical correlation of radiographic mastoiditis on computed tomography (CT) and magnetic resonance imaging (MRI)
Introduction
Acute mastoiditis is a rare otologic emergency and complication of middle ear infection, with suppuration extending to involve the periosteum and bony septa of the mastoid. It is an inflammatory and infective process characterized by demineralization and destruction of the mastoid process of the temporal bone. Mastoiditis has been described as either incipient, in which there is periostitis without osteitis, or alternatively coalescent, where mucoperiosteal disease involves bone and infective osteolysis becomes a cardinal feature (1). Whilst there are no clearly established diagnostic criteria for mastoiditis (2), ultimately it is a clinical diagnosis with otoscopic evidence of middle ear infection and mastoid inflammatory findings, which is often supported by radiologic or surgical findings (3). Clinical signs include auricular proptosis with loss of the postauricular sulcus and postauricular swelling, erythema, and boggy tenderness.
The progression of acute otitis media (AOM) to coalescent mastoiditis results from continued destruction of the mastoid septa and framework (4). This can progress to intratemporal, intracranial, and extracranial complications. Radiological signs of mastoiditis include opacification of the middle ear cleft and mastoid, destruction of the mastoid bony septa and boundaries of the middle ear, local fat stranding, and the presence of rim-enhancing collections (5). Management, either conservative or surgical, is dependent upon clinical and radiological markers of disease severity, particularly suppurative complications.
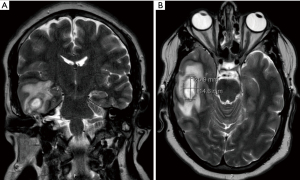
Radiographic mastoid opacification (RMO) is a common, non-specific finding that has been reported in many otologic conditions and as an incidental finding in patients being investigated for non-otologic diseases of the head and neck (Figure 1). Middle ear opacification can represent sterile transudate, infected exudate, blood, or solid material which cannot be reliably differentiated radiographically on computed tomography (CT). RMO may be indiscriminately present in several infective and non-infective otologic pathologies including acute otitis externa (AOE) (6), suppurative and serous otitis media, chronic suppurative otitis media (CSOM) (7), cholesteatoma (8), temporal bone neoplasms (9), and haemotympanum (9). This occurs as pneumatized spaces of the temporal bone, including the middle ear cavity, mastoid, and petrous apex cells, are contiguous and inflammation in one region may cause reactive effusion in another, even in the absence of clinical mastoid infection. Non-otologic pathology described in association with mastoid effusion include radiation toxicity (10), eustachian tube dysfunction (11), sinusitis (12), sinonasal and nasopharyngeal malignancies, upper respiratory tract infections (URTIs), and the presence of endotracheal and nasogastric tubes in critically ill patients (13). Previous studies have described rates of incidental mastoid opacification (IMO) of around 1–5% in adults (11,14-17) and 10–20% in children (18-22).
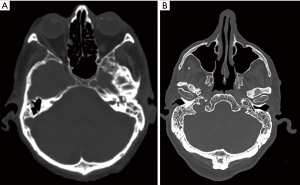
Describing opacification of the mastoid cavity as mastoiditis is hyperbolic and alarming for the non-otolaryngologist. This can lead to unnecessary treatment escalation in healthy patients, including antibiotic therapy, unwarranted investigations, or referral to otolaryngologists. This study aims to determine the clinical correlation of asymptomatic and symptomatic patients with mastoid opacification and radiographic mastoiditis, and investigate what findings should prompt referral to Ear, Nose and Throat (ENT). We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-34/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Nepean Hospital Human Research Ethics Committee (Nos. 2021/STE03843 and 2021/ETH11545) and a waiver of consent was provided. The medical imaging reporting system used by the Nepean Hospital Radiology Department (Sectra Workstation IDS7, Version, Linköping, Sweden) was analyzed to include all CT and magnetic resonance imaging (MRI) studies that included the mastoid cavity and middle ear cleft. This included CT brain, CT brain stroke protocol, CT facial bones and trauma series, CT paranasal sinuses, CT petrous temporal bones, MRI brain, MRI cerebellopontine angle and internal auditory meatus, and MRI orbits.
We utilized an advanced search algorithm using appropriate Boolean operators (“and” and “or”) to identify all CT and MRI scans performed between 1st January 2016 and 28th December 2021 at Nepean Hospital where the report contained the text “mastoid opacification”, “mastoiditis”, or “otomastoiditis”. Inpatient, outpatient, and emergency department scans were included in our study. Our exclusion criteria were patients with known history of cholesteatoma in the ipsilesional ear and no documented follow-up. The indication for imaging was recorded as otologic (i.e., otalgia, otorrhoea, or suspected mastoiditis) or non-otologic. For patients who had multiple craniofacial imaging performed within the study period we only included 1 CT and 1 MRI scan per patient, to avoid inflating the prevalence of RMO and mastoiditis. Similarly, when investigating the prevalence of RMO we counted the total number of unique patients who had craniofacial scans within the study period rather than total scans performed. We define IMO, or incidental mastoid opacification, as RMO in the asymptomatic patient without otologic indication for the scan. We define RMO, or radiographic mastoid opacification, as mastoid opacification demonstrated on imaging. We define clinical mastoiditis as mastoiditis diagnosed in patients with documented evidence of consistent clinical history and/or examination findings, where the diagnosis is not made solely based on the imaging report.
We performed a retrospective chart review of all included patients and analyzed their electronic medical records (Cerner Millenium Powerchart, NSW Health, NSW, Australia). We recorded demographic information, the history and examination findings, the presence of otologic signs or symptoms (OSS), and if they received an ENT review. We determined how many patients with RMO had a clinical diagnosis of mastoiditis or an otologic diagnosis or explanation for the RMO. In patients with clinical mastoiditis, we reviewed their scans to determine what radiographic features were common in these patients.
Data was organized and compiled into Microsoft Excel version 16.53 (Microsoft Corporation, Redmond, WA, USA). All statistical analysis was performed using commercially available software, including Microsoft Excel and IBM SPSS Statistics Version 28.0.1.0 (SPSS Inc., Chicago, Illinois, USA). Continuous data was expressed as mean ± standard deviation. The chi-squared test for independence was used as a test of association to determine whether comparisons between datasets were statistically significant and the odds ratio (OR) was used to measure association. The Pearson’s correlation coefficient was used to determine the degree of linear relationship between two variables. A two-tailed P value <0.05 was used for statistical significance. Categorical data in tables is represented as frequency (n) and percentage of the dataset (%) approximated to 1 decimal place. A multivariable linear regression analysis was performed to identify predictors of clinical diagnosis of mastoiditis and otologic disease in RMO.
Results
Within the study period, there were a total of 13,497 and 35,516 patients who had MRI and CT scans that included the mastoid region. We identified 65 MRI and 238 CT patients where the radiology report mentioned mastoid opacification, representing 0.48% and 0.67% of total scans respectively. Of these, 50 (76.9%) and 192 (80.7%) of the MRI and CT scans were reported as mastoiditis (Figure 2). Using a chi-squared test, the incidence of RMO was significantly higher in the CT cohort compared to MRI (χ2=5.751, P=0.017). Forty-seven scans demonstrated bilateral RMO. The clinical demographics and the indications for imaging are summarized in Tables 2,3. Imaging ordered by ENT surgeons had a higher clinical correlation with otologic disease compared to other physicians, as demonstrated on chi-square test (χ2=55.337, P<0.001). These patients were more likely to be symptomatic and have dedicated cross-sectional imaging. Amongst children (age ≤18 years), 20 patients representing 18 CT and 4 MRI scans had RMO, with bilateral RMO reported in 4. Clinical mastoiditis was diagnosed in 1 patient with a subperiosteal abscess. Due to the small paediatric cohort, meaningful statistical comparison based on patient age could not be performed.
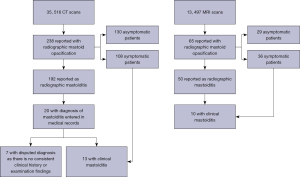
Table 2
| Characteristics | CT cohort (n=238) | MRI cohort (n=65) |
|---|---|---|
| Age (years), mean ± SD | 55±24 | 55±21 |
| Sex, n (%) | ||
| Male | 126 (52.9) | 42 (64.6) |
| Female | 112 (47.1) | 23 (35.4) |
| Paediatric (≤18 years), n (%) | 18 (7.6) | 4 (6.2) |
| Smoker, n (%) | 47 (19.7) | 10 (15.4) |
| Diabetes mellitus, n (%) | 55 (23.1) | 17 (26.2) |
| ATSI origin, n (%) | 24 (10.1) | 5 (7.7) |
| Ordering physician, n (%) | ||
| ENT | 41 (17.2) | 30 (46.2) |
| Emergency Department | 156 (65.5) | 0 (0.) |
| Other subspecialties | 41 (17.2) | 35 (53.8) |
CT, computed tomography; MRI, magnetic resonance imaging; ATSI, Aboriginal and Torres Strait Islander; ENT, Ear, Nose and Throat; SD, standard deviation.
Table 3
| Indication | CT cohort (n=238) | MRI cohort (n=65) |
|---|---|---|
| Otologic symptoms | 108 | 36 |
| Trauma | 38 | 0 |
| Altered level of consciousness | 31 | 3 |
| Suspected stroke or weakness | 20 | 14 |
| Headache | 14 | 5 |
| Seizure | 12 | 3 |
| Vertigo | 7 | 4 |
| Sinonasal symptoms | 2 | 0 |
| Oncosurveillance | 6 | 0 |
CT, computed tomography; MRI, magnetic resonance imaging.
Comparison of symptomatic and asymptomatic populations
One hundred and forty-four scans were performed in patients who had otologic symptoms and 129 (89.6%) were reviewed by ENT. Twelve had no identifiable cause for RMO. Nineteen patients with otologic symptoms representing 28 scans had a diagnosis of mastoiditis documented in the medical records. However, 5 patients presented with non-infective otologic symptoms and without clinical examination findings of middle ear or mastoid infection, and were diagnosed without ENT referral, evidence of bony erosion, or signs consistent with middle ear infection—hence the diagnosis is disputed by the authors following retrospective chart review. In the asymptomatic population, 24 out of 159 patients were reviewed by ENT (15.1%). One-hundred and forty-seven patients had no documented reason for RMO (92.5%), with 3 found to have signs of AOM, 4 with sinusitis, 2 with CSOM, and 2 having radiotherapy. Two patients had a documented diagnosis of mastoiditis in their medical records, which is doubtful as this was based solely on the report and without otologic symptoms, signs, or ENT referral.
The presence of otologic signs and symptoms correlated significantly with clinical mastoiditis [χ2=27.482, OR =1.190, 95% confidence interval (CI): 1.108–1.278, P<0.001] and otologic disease (χ2=214.798, OR =151.000, 95% CI: 62.585–364.320, P<0.001) compared to asymptomatic patients. Mastoid opacification in asymptomatic patients rarely correlated clinically with acute otologic disease.
A summary of the clinical diagnoses for the CT and MRI cohorts is provided in Table 1 and an analysis of radiographic findings and management is reported in Tables 4,5.
Table 1
| Diagnosis | CT cohort (n=238), n (%) | MRI cohort (n=65), n (%) |
|---|---|---|
| No diagnosis | 123 (51.7) | 35 (53.8) |
| AOM | 39 (16.4) | 5 (7.7) |
| AOE | 20 (8.4) | 3 (4.6) |
| NOE | 14 (5.9) | 5 (7.7) |
| CSOM | 10 (4.2) | 3 (4.6) |
| Mastoiditis | 20 (8.4) | 10 (15.4) |
| Sinusitis or URTI | 5 (1.7) | 2 (3.1) |
| Cholesteatoma | 3 (1.3) | 0 (0) |
| Other | 4 (1.7) | 2 (3.1) |
Whilst a total of 21 patients representing 20 CT and 10 MRI scans had a diagnosis of mastoiditis documented in the medical records, the diagnosis is disputed by the authors in 7 patients where the diagnosis was made purely based on the radiology report and without consistent history or examination findings, ENT referral, or radiographic evidence of bony destruction. RMO, radiographic mastoid opacification; CT, computed tomography; MRI, magnetic resonance imaging; AOM, acute otitis media; AOE, acute otitis externa; NOE, necrotising otitis externa; CSOM, chronic suppurative otitis media; URTI, upper respiratory tract infection; ENT, Ear, Nose and Throat.
Table 4
| Variables | CT cohort (n=238), n (%) | MRI cohort (n=65), n (%) |
|---|---|---|
| Ordering physician | ||
| ENT | 41 (17.2) | 30 (46.2) |
| Emergency Department | 155 (65.1) | 0 (0) |
| Other medical or surgical specialties | 42 (17.6) | 35 (53.8) |
| Bony erosion | 39 (16.4) | 18 (27.7) |
| Suppurative complications | 9 (3.8) | 8 (12.3) |
| Paranasal sinus opacification | 81 (34.0) | 18 (27.7) |
| Otologic signs or symptoms | 108 (45.4) | 36 (55.4) |
| ENT referral in symptomatic patients | 96 (88.9) | 33 (91.7) |
| Asymptomatic cohort | 130 (54.6) | 29 (44.6) |
| ENT referral in asymptomatic cohort | 21 (16.2) | 3 (10.3) |
| Antibiotic treatment | 100 (42.0) | 24 (36.9) |
| Surgical intervention | 19 (8.0) | 12 (18.5) |
CT, computed tomography; MRI, magnetic resonance imaging; ENT, Ear, Nose and Throat.
Table 5
| Management | Number |
|---|---|
| Myringotomy | 7 |
| Ventilation tube (grommet) insertion | 6 |
| Cortical mastoidectomy | 6 |
| Elective mastoidectomy | 2 |
| Drainage of cerebral abscess | 2 |
| External ventricular drain insertion | 2 |
| Modified radical mastoidectomy | 1 |
| Drainage of subperiosteal abscess | 1 |
Analysis of CT and MRI cohort
Within the 238 CT scans, bony erosion was noted in 39 patients, with infective pathology diagnosed in patients with mastoiditis (n=13), AOM (n=7) including complicated AOM (n=3), and CSOM (n=2). Whilst 20 patients in the CT cohort had a diagnosis of mastoiditis documented in the clinical notes, 7 of these diagnoses were made without examination findings, bony erosion, or suppurative complications, and were based purely on the radiologist impression and without ENT referral—hence, the authors dispute the diagnosis in these patients. One hundred patients received antibiotic treatment for ear infection and 19 had surgical intervention for mastoiditis (n=12), complicated AOM (n=3), cholesteatoma (n=2), CSOM (n=1), and necrotising otitis externa (NOE) (n=1). A proportion of 91.4% (n=53/58) of patients who had CT petrous temporal bones had an otologic diagnosis, as they were often ordered by ENT surgeons and due to otologic presentation.
The 65 MRI scans with RMO were only ordered by ENT surgeons (n=30) or other subspecialties (n=35) including Neurology, Neurosurgery, or Infectious Diseases, with no scans ordered by the Emergency Department. Of the 18 patients with bony erosion, the clinical diagnosis included mastoiditis (n=10), NOE with skull base osteomyelitis (n=4), new diagnosis of attic cholesteatoma (n=1), mastoid-cutaneous fistula from osteoradionecrosis (n=1), AOM (n=1), and CSOM (n=1). Twenty-four patients received antibiotic treatment for ear infection and 12 had surgical intervention for mastoiditis (n=9), complex AOM (n=2), and mastoid-cutaneous fistula (n=1). Whilst MRI is not the optimal modality for assessing bony erosion, it was noted in 18 patients in the MRI cohort.
Analysis of clinical mastoiditis cohort
Fourteen patients had documented clinical evidence of acute mastoiditis, including 1 child and 13 adults. This represented 13 CT and 10 MRI scans. All patients had otologic and/or neurologic symptoms and positive examination findings, with bony erosion on cross-sectional imaging. Twelve patients had suppurative complications including otogenic meningitis (n=6), otitic hydrocephalus (n=2), and 1 instance each of temporal lobe abscess, subdural empyema, petrous apicitis, ventriculitis, subperiosteal abscess, Bezold abscess, and sigmoid sinus thrombosis. Ten patients underwent emergency surgical management, all in the cohort with suppurative complications, which is summarized in Table 5. The others had conservative management with intravenous antibiotic therapy, with two having elective mastoidectomy for cholesteatoma and CSOM refractory to maximal medical therapy. A summary of the mastoiditis patients is provided in Table S1. Representative imaging from the mastoiditis cohort is provided in Figures 3-5 showing evidence of bony erosion and/or suppurative complications.
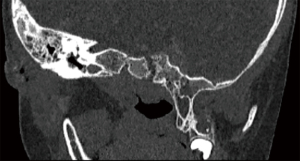
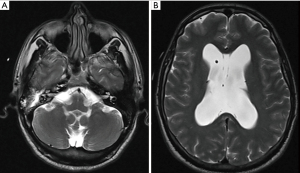
The presence of bony erosion significantly correlated with mastoiditis compared to the absence of bony erosion, with χ2=98.381, OR =159.716 (95% CI: 20.784–1219.090, P<0.001). Similarly, the presence of suppurative complications strongly correlated with mastoiditis with χ2=90.075 OR =50.417 (95% CI: 15.112–168.199, P<0.001). The Pearson’s rank correlation coefficient between radiologic and clinical mastoiditis was insignificant in the CT (r=0.066, P=0.313) and MRI (r=0.234, P=0.061) cohorts, and weak in the total cohort (r=0.147, P=0.010). The P value result in the total cohort is likely due to the larger sample size (23). When considering the symptomatic cohort only, the Pearson’s rank correlation coefficient between radiologic and clinical mastoiditis was insignificant in the CT cohort (r=0.181, P=0.060) and weakly significant in the MRI cohort (r=0.358, P=0.032).
A multivariable linear regression analysis was performed for the dependent variables of clinical mastoiditis and clinical diagnosis of otologic disease, with the independent variables of gender, physician who ordered the scan, RMO, radiographic bony erosion, radiographic evidence of suppurative complications, radiographic paranasal sinus opacification, and presence of OSS at presentation. For clinical mastoiditis, in the MRI cohort only the presence of bony erosion (P<0.001) and suppurative complications (P=0.007) were significant predictors; in the CT cohort, the presence of bony erosion (P<0.001), suppurative complications (P<0.001), and OSS (P=0.033) were significant predictors; and when considering both CT and MRI cohorts together the presence of bony erosion (P<0.001), suppurative complications (P<0.001), and OSS (P=0.023) were significant predictors. When considering the clinical diagnosis of otologic disease, in the MRI cohort, the ordering physician (P=0.037) and presence of OSS (P<0.001) were significant predictors; in the CT and the combined CT and MRI cohorts only OSS (P<0.001 and P<0.001 respectively) was a significant predictor.
Discussion
Summary of main results
The findings of our study demonstrates that mastoiditis is rare in the setting of isolated RMO without bony erosion, and requires the presence of positive otologic symptoms and signs. In the asymptomatic cohort with IMO, there were no cases of mastoiditis. RMO in the symptomatic population was present in various otologic pathologies, with the majority being benign, uncomplicated ear infections. The majority of these diagnoses such as AOE and AOM do not specifically require ENT referral. Thirteen out of 108 (12.0%) and 10 out of 36 (27.8%) symptomatic patients in the CT and MRI cohorts respectively had a clinical diagnosis of mastoiditis. However, the main distinction is that each patient had demonstrable erosive bone changes rather than isolated mastoid opacification and this should be the imaging finding that raises suspicion of mastoiditis. It is important to recognize that the vast majority of AOE and AOM, who likely would have had some degree RMO, would not have had imaging, and hence the proportion of symptomatic patients with RMO diagnosed with clinical mastoiditis would be inflated, particularly in the MRI cohort. Erosive findings and suppurative complications correlated with mastoiditis, but were also seen in cases of complex AOM or NOE. ENT referral is warranted in these patients to commence medical or surgical management of temporal bone infection and prevent complications.
The clinical correlation of RMO with otologic disease was higher in the MRI cohort compared to the CT cohort, with a weak, yet significant correlation, with clinical mastoiditis in symptomatic patients. All MRI scans were ordered by ENT or other sub-specialties, usually as a second-line investigation in patients with convincing otologic or neurologic symptoms and often based on concerning CT findings. Hence, MRI scans had a higher pre-test probability of demonstrating sinister pathology including mastoiditis and NOE with skull base osteomyelitis. CT scans that reported RMO were often ordered by Emergency Department physicians for otologic and non-otologic reasons in an otherwise undifferentiated patient. Our data also shows that mastoiditis was incorrectly entered as a diagnosis in the medical records by junior doctors based purely on the radiology report, without supporting clinical evidence. This demonstrates that inappropriate reporting of RMO as mastoiditis can lead to misleading diagnoses and unnecessary treatment by the non-otolaryngologist. RMO was a poor correlate to clinical disease in the asymptomatic population.
Comparison with existing literature
Previous studies have reported variable rates of RMO in adult, paediatric, and asymptomatic cohorts. A meta-analysis of 15 studies by Mughal et al. showed that the pooled prevalence of IMO was 8.44% (24). This figure is likely inflated by studies with low total scans, as our prevalence of 0.48% in MRI and 0.67% in CT is closer to large volume studies such as Sayal (n=205,792, 0.14%), Polat (n=29,850, 1.36%), Wilkinson (n=15,050, 0.41%), Orhan (n=2,270, 0.44%), and Pastuszek (n=1,927, 1.71%) (11,12,14,25,26). Marked heterogeneity was present in the meta-analysis which was partially attributable to imaging modality (CT or MRI) evaluated in the study, age group of patient population, sample size, and method of measuring IMO. The pooled prevalence in children was higher compared to adults, 17.16% versus 6.09% (24). Studies have demonstrated higher IMO prevalence in children (19), likely due to anatomic factors predisposing to Eustachian tube dysfunction, increased incidence of AOM or URTI, and relative mastoid hypopneumatization compared to adults. Mastoid opacification was more prevalent in studies that directly reviewed the scans compared to those that used the radiologist report, 14.52% versus 3.50% (24). This suggests that IMO may be even more common in asymptomatic populations as it is the discretion of the radiologist whether it is reported. This reinforces the notion that diagnosing mastoiditis based entirely on the report without clinical context is inappropriate due to variability in reporting preferences amongst radiologists. RMO is inconsistently defined amongst studies and can include partial or total opacification. In an MRI study, Saat et al. showed that most patients with acute mastoiditis had ≥50% involvement of the tympanum and 100% of the mastoid antrum and air cells (27).
Previous studies largely focussed on RMO in asymptomatic patients and excluded those with otologic indications for imaging. Pastuszek reported 3 of 52 patients with RMO had clinical evidence of mastoiditis (12). In a study of 27 patients with AOE, Brennan et al. showed that 79% had fluid in the mastoid or tympanum on CT petrous temporal bones, without clinical evidence of middle ear infection (6). This probably reflects inflammatory spread between neighbouring anatomic spaces. However, as most cases of AOE are uncomplicated and without need for imaging, it is possible that RMO in AOE reflects disease severity. Pastuszek reported that 17.3% patients with CT evidence of RMO were diagnosed with AOE, compared to 8.4% in our study (12). Currently there is no data on how long RMO persists in either symptomatic or asymptomatic patients.
An experimental animal model in monkeys showed that middle ear insufflation with CO2 gas generated negative middle ear pressure (NMEP), confirmed on tympanometry, with T2-signal mastoid hyperintensity suggestive of effusion (28). Hence, incidental mastoid effusions may represent periodic cycling of middle ear pressures, and a physiologic explanation is suggested by our data where the overwhelming cases of RMO in asymptomatic populations had no obvious or identifiable pathologic cause.
Role of temporal bone imaging in diagnosing mastoiditis
Bony erosion of the mastoid septa, cortex, and boundaries of the mastoid and middle ear cavity is the hallmark of coalescent mastoiditis. Mastoid infection can subsequently extend into the temporal bone, intracranial compartment, or perimastoid soft tissues of the neck (29,30). CT remains the first-line investigation in the diagnosis of suspected mastoiditis due to accessibility and familiarity. High-resolution dedicated imaging of the petrous temporal bones is important for optimal bony detail (31), with high sensitivity for erosive findings (32), whereas non-dedicated imaging such as CT brain may show RMO but fail to detect bony changes due to thicker slices. Erosive findings should be interpreted carefully as they may represent underlying cholesteatoma or CSOM (5). Tegmen tympanum dehiscence is important to evaluate to determine the risk of intracranial spread, though meningitis may still occur in its absence. Contrast-enhanced studies are useful to assess perimastoid soft tissue inflammation, rim-enhancing collections, and venous sinus thrombosis (27). MRI is useful for suspected intracranial involvement and can provide additional radiologic markers of mastoiditis including major intramastoid signal changes relative to cerebrospinal fluid and white matter, diffusion restriction, or abnormal enhancement of mastoid mucosa, periosteum, or dura (27). MRI is sensitive to visualising edema and inflammation, and T2-weighted mucosal enhancement within the mastoid will be seen as a bright field against the dark background of the surrounding pneumatized temporal bone (33). Platzek showed a 100% sensitivity and 66% specificity in confirming diagnosis in 23 patients with suspected mastoiditis with the presence of 2 of the following intramastoid findings: fluid accumulation, enhancement, or diffusion restriction (34). However, this is not generalizable to the asymptomatic population or those with no clinical signs of mastoid inflammation. In addition, MRI has limitations in detecting bony destruction.
Increased utilization of cross-sectional imaging and advances in imaging techniques and their sensitivity may lead to increased detection of incidental and clinically insignificant findings (25). Diagnosing mastoiditis based on RMO on CT or intramastoid signal intensity on MRI alone, which simply suggests mastoid fluid, is inappropriate without clinical context. Whilst isolated mastoid effusion can represent incipient mastoiditis in the precoalescent stage, in the absence of otologic symptoms or otoscopic signs of AOM this is rare.
Study strengths and limitations
Our study includes a large volume of scans over a long time period compared to previous studies within the field. This increases the generalizability of our data. Whereas previous studies generally excluded symptomatic patients, our data includes patients with and without otologic symptoms and signs. This allowed us to compare the clinical utility of RMO in both populations. We included both CT and MRI imaging, allowing us to make comparisons between modalities. Our results have high external validity and usefulness for non-otolaryngologist physicians in making clinical decisions amongst various types of patients.
In their meta-analysis of studies on mastoid opacification, Mughal et al. showed that studies where the authors reviewed the scans reported a higher prevalence compared to those that only read the radiologist’s report (24). Hence, it is possible that our data underestimates the true prevalence of RMO. Regardless, the data adequately addresses the study aim which is to address whether radiologist reporting of mastoiditis correlates with clinical mastoiditis or other otologic disease.
Our study has a small paediatric cohort, a population in which otologic disease and mastoid opacification is more common, potentially underestimating RMO prevalence. This is because children with complicated pathology are generally referred to tertiary paediatric institutions in our health district. As such imaging of paediatric patients is uncommonly performed at our facility.
Future studies in this area could focus on the interobserver reliability of detecting RMO and reporting it as mastoiditis amongst radiologists, comparisons between interpretation of RMO between radiologist and otolaryngologist, and determining the utility of artificial intelligence algorithms in predicting mastoiditis.
Conclusions
Mastoid opacification can be found in both symptomatic and asymptomatic populations, is present in various otologic and non-otologic disease states, and has poor clinical correlation with mastoiditis, which in our experience is over-reported on imaging. A weak correlation between clinical and radiologic mastoiditis was seen in symptomatic patients on MRI, suggesting that it is useful as a second-line modality in the diagnostic workup of patients with high pre-test probability of mastoiditis and suspicion of intracranial complications. Whilst reporting mastoid opacification should not be discouraged, we suggest radiologists adopt the term mastoid opacification or effusion rather than ‘mastoiditis’ in the absence of erosive findings or suppurative complications, to prevent over-treatment, anxiety amongst patients and clinicians, and allow appropriate triage of referrals to ENT services.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-22-34/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-22-34/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-22-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-34/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study received approval by the Nepean Hospital Human Research Ethics Committee as a low or negligible risk project (2021/STE03843 and 2021/ETH11545) and a waiver of consent was provided.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Budenz C, El-Kashlan H. Complications of Temporal Bone Infections. In: Flint P, Francis H, Haughey B, et al. Cummings Otolaryngology. 7th edition. Philadelphia: Elsevier, 2020:2135-54.
- van den Aardweg MT, Rovers MM, de Ru JA, et al. A systematic review of diagnostic criteria for acute mastoiditis in children. Otol Neurotol 2008;29:751-7. [Crossref] [PubMed]
- Luntz M, Brodsky A, Nusem S, et al. Acute mastoiditis--the antibiotic era: a multicenter study. Int J Pediatr Otorhinolaryngol 2001;57:1-9. [Crossref] [PubMed]
- Vazquez E, Castellote A, Piqueras J, et al. Imaging of complications of acute mastoiditis in children. Radiographics 2003;23:359-72. [Crossref] [PubMed]
- Mather MW, Yates PD, Powell J, et al. Radiology of acute mastoiditis and its complications: a pictorial review and interpretation checklist. J Laryngol Otol 2019;133:856-61. [Crossref] [PubMed]
- Brennan TE, Saadia-Redleaf MI. Occult middle ear and mastoid fluid in acute otitis externa. Laryngoscope 2012;122:2067-70. [Crossref] [PubMed]
- Bhalla AS, Singh A, Jana M. Chronically Discharging Ears: Evalution with High Resolution Computed Tomography. Pol J Radiol 2017;82:478-89. [Crossref] [PubMed]
- Más-Estellés F, Mateos-Fernández M, Carrascosa-Bisquert B, et al. Contemporary non-echo-planar diffusion-weighted imaging of middle ear cholesteatomas. Radiographics 2012;32:1197-213. [Crossref] [PubMed]
- Lo AC, Nemec SF. Opacification of the middle ear and mastoid: imaging findings and clues to differential diagnosis. Clin Radiol 2015;70:e1-e13. [Crossref] [PubMed]
- Walker GV, Ahmed S, Allen P, et al. Radiation-induced middle ear and mastoid opacification in skull base tumors treated with radiotherapy. Int J Radiat Oncol Biol Phys 2011;81:e819-23. [Crossref] [PubMed]
- Polat S, Aksoy E, Serin GM, et al. Incidental diagnosis of mastoiditis on MRI. Eur Arch Otorhinolaryngol 2011;268:1135-8. [Crossref] [PubMed]
- Pastuszek A, Lomas J, Grigg C, et al. Is mastoiditis being over-diagnosed on computed tomography imaging? —radiological versus clinical findings. Aust J Otolaryngol 2020;3:31. [Crossref]
- Huyett P, Raz Y, Hirsch BE, et al. Radiographic Mastoid and Middle Ear Effusions in Intensive Care Unit Subjects. Respir Care 2017;62:350-6. [Crossref] [PubMed]
- Orhan K, Nishiyama H, Tadashi S, et al. MR of 2270 TMJs: prevalence of radiographic presence of otomastoiditis in temporomandibular joint disorders. Eur J Radiol 2005;55:102-7. [Crossref] [PubMed]
- Orhan K, Avsever H, Aksoy S, et al. Temporomandibular joint MR images: Incidental head and neck findings and pathologies. Cranio 2019;37:121-8. [Crossref] [PubMed]
- Makdissi J, Pawar RR, Radon M, et al. Incidental findings on MRI of the temporomandibular joint. Dentomaxillofac Radiol 2013;42:20130175. [Crossref] [PubMed]
- Abbas Y, Yuen HS, Trinidade A, et al. Incidental mastoiditis on magnetic resonance imaging scans: clinical relevance and cost implications. J Laryngol Otol 2018;132:1010-2. [Crossref] [PubMed]
- Lafferty DJ, Cohn JE, McKinnon BJ. Incidental mastoid opacification on computed tomography in the pediatric population. Int J Pediatr Otorhinolaryngol 2020;128:109688. [Crossref] [PubMed]
- Placanica T, Anderson S. Incidental finding of mastoid opacification in computed tomography imaging of the asymptomatic paediatric population. J Laryngol Otol 2019;133:588-91. [Crossref] [PubMed]
- Singh S, Rettiganti MR, Qin C, et al. Incidental mastoid opacification in children on MRI. Pediatr Radiol 2016;46:704-8. [Crossref] [PubMed]
- von Kalle T, Fabig-Moritz C, Heumann H, et al. Incidental findings in paranasal sinuses and mastoid cells: a cross-sectional magnetic resonance imaging (MRI) study in a pediatric radiology department. Rofo 2012;184:629-34. [Crossref] [PubMed]
- Blomgren K, Robinson S, Lönnqvist T, et al. Clinical significance of incidental magnetic resonance image abnormalities in mastoid cavity and middle ear in children. Int J Pediatr Otorhinolaryngol 2003;67:757-60. [Crossref] [PubMed]
- Demidenko E. The p-Value You Can't Buy. Am Stat 2016;70:33-8. [Crossref] [PubMed]
- Mughal Z, Charlton AR, Clark M. The Prevalence of Incidental Mastoid Opacification and the Need for Intervention: A Meta-Analysis. Laryngoscope 2022;132:422-32. [Crossref] [PubMed]
- Sayal NR, Boyd S, Zach White G, et al. Incidental mastoid effusion diagnosed on imaging: Are we doing right by our patients? Laryngoscope 2019;129:852-7. [Crossref] [PubMed]
- Wilkinson SL, Sahota RS, Constable JD, et al. Does incidental mastoid opacification on computerized tomography necessitate referral to ENT? Laryngoscope 2017;127:2860-5. [Crossref] [PubMed]
- Saat R, Laulajainen-Hongisto AH, Mahmood G, et al. MR imaging features of acute mastoiditis and their clinical relevance. AJNR Am J Neuroradiol 2015;36:361-7. [Crossref] [PubMed]
- Alper CM, Swarts JD, Doyle WJ. Prevention of otitis media with effusion by repeated air inflation in a monkey model. Arch Otolaryngol Head Neck Surg 2000;126:609-14. [Crossref] [PubMed]
- Dubey SP, Larawin V, Molumi CP. Intracranial spread of chronic middle ear suppuration. Am J Otolaryngol 2010;31:73-7. [Crossref] [PubMed]
- Yorgancilar E, Akkus Z, Gun R, et al. Temporal bone erosion in patients with chronic suppurative otitis media. B-ENT 2013;9:17-22. [PubMed]
- Juliano AF. Cross Sectional Imaging of the Ear and Temporal Bone. Head Neck Pathol 2018;12:302-20. [Crossref] [PubMed]
- Migirov L. Computed tomographic versus surgical findings in complicated acute otomastoiditis. Ann Otol Rhinol Laryngol 2003;112:675-7. [Crossref] [PubMed]
- Meredith JR, Boyev KP. Mastoiditis on MRI: Fact or Artefact. Ear Nose Throat J 2008;87:514-8. [Crossref] [PubMed]
- Platzek I, Kitzler HH, Gudziol V, et al. Magnetic resonance imaging in acute mastoiditis. Acta Radiol Short Rep 2014;3:2047981614523415. [Crossref] [PubMed]
Cite this article as: Ananthapadmanabhan S, Jabbour J, Ayeni F, King G, Sritharan N, Sivapathasingam V. Clinical correlation of radiographic mastoiditis on computed tomography (CT) and magnetic resonance imaging (MRI). Aust J Otolaryngol 2023;6:3.


