
Mycobacterium simiae: a rare cause of cervical lymphadenitis—case report
Introduction
Nontuberculous mycobacteria (NTM) are acid-fast organisms that can be classified as either rapid-growing or slow-growing pathogens. There exist over 140 species that cause a range of infections in both immunocompetent and immunosuppressed hosts (1-4). Of these, NTM cervical lymphadenitis is the most common manifestation in immunocompetent children and slowly-growing Mycobacterium avium complex (MAC) is the most common causative pathogen, though other rarer causes have been reported (1,2,4,5). Mycobacterium simiae (M. simiae) is a slow-growing, multidrug-resistant obligate aerobe that generally causes pulmonary, intra-abdominal and occasionally disseminated infections in immunocompromised hosts, within a restricted geographic distribution. This organism is generally difficult to identify, and is also difficult to treat as it is one of the most highly multidrug resistant organisms in the mycobacterium species. Complete surgical resection remains the gold standard of therapy for all NTM cervical lymphadenitis infections, while choice of antibiotic regime remains a difficult decision due to a lack of clinical trials as a result of the low incidence of infection with M. simiae (6-10). We present the following case in accordance with the CARE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-21-50/rc).
Case presentation
A previously healthy 7-year-old Indigenous Australian female was seen by a general practitioner (GP) for a 3-month history of increasing swelling and skin changes over the right side of her neck. The patient denied having fevers, night sweats or weight loss. There was no history of preceding upper respiratory or dental infection. The patient took no regular medications and had no known allergies. The patient had recently moved into foster care and the previous history of exposure to unclean water, or animals was unclear.
Two months prior to seeing this GP, the patient had an ultrasound (US) study of her neck to investigate a swollen right submandibular region. This study showed right submandibular lymphadenopathy with 2 enlarged soft lymph nodes, measuring 2.4 cm × 2.2 cm. A fine needle aspirate (FNA) of the enlarged lymph nodes could not be performed at the time of the US study due to lack of patient cooperation.
She was referred by the GP to the ear, nose and throat (ENT) specialists at the local regional tertiary care centre. Physical examination of the child revealed a well-looking child in no apparent distress. Vital signs were within normal limits and her growth was appropriate for age. On examination of the neck, a discrete 3 cm fluctuant mass was palpable over levels Ib and II. The overlying skin was intact but thinned, and erythematous with violaceous discolouration. Examination of the ears and nose were normal as was the remainder of her physical examination.
Laboratory evaluation revealed a full blood count with normal white blood cell, neutrophil, lymphocyte, monocyte, eosinophil and basophil counts. The haemoglobin and haematocrit were normal, and the platelet count was unremarkable. The C-reactive protein was less than 2.9 mg/dL. Liver function tests and biochemistry were unremarkable. Serology for toxoplasmosis was negative. Serology for both Epstein Barr virus and cytomegalovirus IgG were reactive, and IgM was not detected, suggesting past infection.
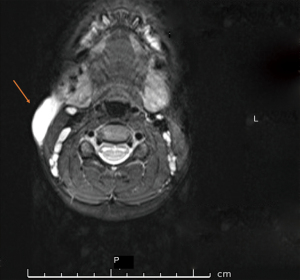
FNA of the lymph node was performed under sedation. 4 mL of cloudy haemoserous fluid was drained from the neck mass and sent for microscopy, culture, and sensitivity (MCS). Analysis of the aspirate did not yield any results and a pre-operative work-up was undertaken including a repeat US scan and magnetic resonance imaging (MRI) scan of the neck. The US revealed progression of lymph node enlargement while the MRI showed a thin walled peripherally enhancing lesion within the superficial soft tissues over the right sternocleidomastoid muscle, extending from just below the level of the right parotid gland to the level of C5 vertebral body (Figure 1). Multiple mildly prominent lymph nodes were seen throughout the neck, but none were pathologically enlarged or necrotic.
The patient underwent incision and drainage of the right neck mass. Necrotic debris was curetted and collected for pathology. The skin easily tore and was unable to be closed primarily and was therefore left to heal by secondary intention. Histology showed fragmented inflammatory tissue with heavy lymphohistiocytic infiltrate and broad zones of fine eosinophilic necrosis with neutrophil debris and dystrophic calcification, features consistent with necrotising granulomatous and mycobacterial infection. Special stains did not however yield any mycobacterial or fungal organisms. Following surgery, the child was commenced on a 5-day course of oral amoxicillin-clavulanate 540 mg twice daily to cover for possible bacterial infection. She was reviewed by the Infectious Diseases service and commenced on a planned 6-month course of clarithromycin 180 mg twice daily and rifampicin 180 mg twice daily for presumed MAC cervical lymphadenitis. For reasons unknown, rifampicin and clarithromycin were only continued for 2 weeks and the patient was lost to follow-up.
Five months post procedure, the patient returned for review by ENT and infectious diseases. The right neck mass was still prominent with overlying erythema and the surgical wound from the incision and drainage had healed with extensive scarring and keloid features. Extended cultures had not yielded any pathogens. The child was again commenced on empirical treatment for MAC infection with clarithromycin 380 mg daily divided into twice daily doses, and rifampicin 190 mg twice daily. She was reviewed regularly by both Paediatric and Infectious Diseases services to ensure medication compliance and tolerance. She reported no side effects and completed a 6-month course of antimicrobial therapy. A repeat US was performed which revealed a 1.9 cm heterogenous hyperaemic structure deep to platysma and posterolateral to her submandibular saliva gland. The surgical team favoured avoiding a nodal dissection due to the significant risk to the marginal branch of her facial nerve. Medical management was therefore continued with Ethambutol 400 mg daily added to the antibiotic regimen due to suboptimal response to dual-agent therapy. A chest radiograph was clear with no evidence of pulmonary infiltrates. Baseline ophthalmology assessment was performed and repeated at 3 and 6 months to monitor for signs of optic neuropathy secondary to ethambutol. Triple-antibiotic therapy was continued for an additional 6 months. At the 11th month of medical management, she presented with minor skin breakdown overlying the cervical node with associated intermittent wound discharge.
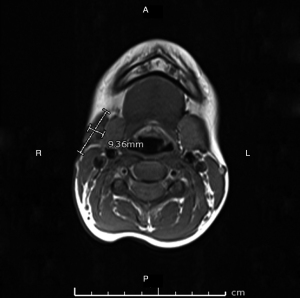
Discharge from the wound was swabbed and sent for MCS, acid-fast bacilli (AFB) smear and panmycobacterial PCR which were negative, and from a multidisciplinary team opinion, definitive surgical management was now required. Surgical work-up included a repeat MRI which revealed persistent lobulated and heterogenous tissues at the site of the previous collection superficial to the right submandibular gland abutting the inferior aspect of the body of mandible, measuring 2.2 cm × 0.7 cm × 2.7 cm (transverse, anterior-posterior, superior-inferior), with smaller rounded regions of T2 and T1 hypointensity and little peripheral enhancement (Figure 2). Reactive lymph nodes persisted within the deep cervical lymph chain and posterior triangle of the neck.
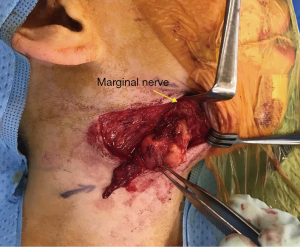
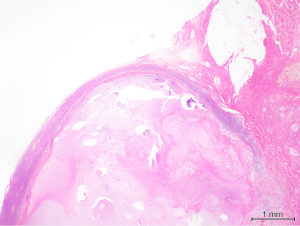
Surgical excision of the right submandibular lymph nodes and revision of scar was undertaken. Intraoperatively both the marginal mandibular and cervical branch of her facial nerve were located and seen to be running directly over an enlarged and fluctuant right perifacial node (Figure 3). The nodal mass was dissected off the submandibular gland once the facial nerve branches had been mobilised and preserved. The resected tissue was bisected and sent fresh and in formalin to pathology for histology, MCS, AFB, panmycobacterial PCR and pan fungal PCR. Histopathology revealed granulomatous inflammation with a large central area of necrosis, degenerate material and a thin rim of fibrous stroma surrounding the area of necrosis and residual rim of lymphoid tissue (Figure 4). Scattered chronic inflammatory cells and some giant cells with few non-necrotising granulomas were seen in the adjacent fibroadipose tissue. Special stains did not yield any mycobacterial organisms and there was no evidence of atypical cells, malignancy or fungal organisms. Culture from nodal tissue grew Staphylococcus epidermidis and PCR identified M. simiae.
The child recovered well post-operatively with no palpable adenopathy. Some mild right-sided mandibular facial nerve palsy was evident but improved on outpatient review eight weeks post-operatively. Five months post-operatively the child was clinically well with a healed wound and no palpable cervical adenopathy. An US of the neck revealed non-pathological small lymphadenopathy without central necrosis.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parent or legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal on request.
Discussion
NTM are acid-fast organisms that reside in water and soil. There are currently more than 140 recognised species of NTM, classified as rapid-growing or slow-growing pathogens. They can cause a broad range of infections including skin and soft tissue infections (SSTIs), lymphadenitis, pulmonary disease, otitis media and osteomyelitis (1-4,11). In a prospective national study of NTM infection in children in Australia, Blyth et al. [2009] found that disseminated disease and mediastinal lymphadenitis are atypical presentations, and are more likely to be associated with underlying immunodeficiency (12). Additionally, pulmonary infection, though not atypical, is more likely to occur in association with underlying lung disease. Cervical lymphadenitis is the most common manifestation in immunocompetent children and slow-growing MAC is the most common causative pathogen followed by M. scrofulaceum, M. kansasii, M. fortuitum and M. haemophilum (1,2,4,5). Table 1 outlines the available case reports of NTM presentations in the paediatric population since 1989 in Australia and New Zealand. Notably, the species isolated in each report is variable with some cases identifying multiple NTM species, and there appears to be no consistent mode of treatment amongst reports (1,13-28). None of these case reports identified a case of M. simiae. Due to their slowly-growing nature, NTM cervical lymphadenitis typically presents as a non-tender slowly enlarging unilateral neck mass in the submandibular, parotid and pre-auricular regions with violaceous skin discolouration secondary to inflammatory reactions (6,7). Over several months, the enlarging mass can undergo central necrosis, liquefaction, fluctuation, eruption and sinus formation and at this stage most children are already referred to or seen by an ENT or paediatric infectious disease specialist (1,4,6).
Table 1
| Author | Year of publication | Patient demographics | Pathogens isolated | Management |
|---|---|---|---|---|
| Joshi et al. (13) | 1989 | Review of 86 children | Unspecified isolates | All children were treated surgically and recurrence occurred in five patients |
| Pang (14) | 1992 | Review of 118 children | M. tuberculosis, avium complex and scrofulaceum | Total excision with 10% of patients requiring second excision due to relapse or residual disease |
| Goutzamanis and Gilbert (15) | 1995 | Review of 8 children | M. ulcerans | Surgical excision for all patients with one patient requiring limb saving heat treatment after both anti-mycobacterial drug and surgery failed to stop progression of necrotizing ulceration |
| Wright (16) | 1996 | Review of 89 children ages 1 to 10 years old | Unspecified NTM isolates | 55 surgical excisions with one recurrence and eight excision and curettage with two recurrences |
| Wark et al. (17) | 1998 | Review of 10 children ages 1 to 5 years old | M. avium, intracellulare, gordanae | Nine surgical excisions as initial management with one developing a discharging sinus requiring 2 weeks of Trimethoprim/Sulfamethoxazole and Rifampicin |
| 1 incision and drainage with recurrence of disease requiring complete excision | ||||
| Fergusson and Simpson (18) | 1999 | Review of 10 children | Unspecified NTM isolates | Ten successful curettages with two experiencing delayed wound healing and one requiring a repeat curettage 7 months post-primary excision due to recurrence |
| O’Brien et al. (19) | 2000 | Review of 4 children ages 0 to 9 years old | M. avium complex, fortuitum, gordanae | All 4 underwent surgical excision for lymphadenitis with no adjunctive chemotherapy and no relapse of disease detected |
| Flint et al. (20) | 2000 | Review of 57 children | M. avium intracellulare, kansasaii | Eleven received surgical excisions, 30 patients received incision and drainage, 13 received incision and curettage and three had aspirations |
| Daley (11) | 2001 | 3-year-old patient | M. avium complex | Spontaneous resolution of lymphadenopathy, no medical or surgical intervention |
| Blyth et al. (12) | 2009 | Review of 102 children ages 1 to 14 years old | M. avium complex, intracellulare | 78 surgical procedures and 42 received anti-mycobacterial therapy, 25 received both therapies |
| Sparks and Khatami (21) | 2014 | 14-year-old patient | M. fortuitum | 10-week course of oral Trimethoprim/Sulfamethoxazole and Moxifloxacin |
| Chong et al. (22) | 2015 | 12-year-old patient | M. avium complex, intracellulare | 12-month course of Ethambutol and Clarithromycin |
| Tebruegge M et al. (1) | 2016 | Review of 140 children with NTM disease | M. avium complex, ulcerans, marinum | 97.2% of lymphadenitis cases underwent surgical excision with reduced disease recurrence in groups treated with Clarithromycin and Rifampicin compared with groups with Clarithromycin alone or no anti-mycobacterial drugs |
| Mahadevan (23) | 2016 | Review of 97 children ages 8 to 15 years old | Unspecified NTM isolates | Higher cure rates with excision compared to incision and drainage |
| Freyne and Curtis (24) | 2017 | 3-year-old patient | M. gordonae | Excision biopsy followed by treatment of Clarithromycin, Rifampicin and Ethambutol for 3 months. Clarithromycin changed to Azithromycin for improved compliance and oral regimen continued for 6 months total |
| Berkhout A et al. (25) | 2020 | 13-year-old patient | M. abscessus | Initial treatment with two surgical debridements and insertion of Vancomycin beads followed by 8-week oral course of Azithromycin and Linezolid followed by 6-month course of same while awaiting definitive procedure |
| Aliano and Thomson (26) | 2020 | Review of 99 children ages 0 and 12 years old | M. avium complex, intracellulare, haemophilum | Not discussed in article |
| Foley et al. (27) | 2021 | 12-year-old patient | M. fortuitum | Initial treatment with surgical debridement, oral Rifampicin and Doxycycline. Changed to intravenous Meropenem and oral Azithromycin, Doxycycline, Ciprofloxacin and Fluconazole on day 6. Antimicrobials rationalised to Doxycycline, Ciprofloxacin and Fluconazole on day 30 |
| Weng et al. (28) | 2022 | 20-month-old patient | M. avium complex | Unspecified in abstract |
NTM, nontuberculous mycobacteria.
In our case, M. simiae was detected on PCR. M. simiae is a slow-growing, multidrug-resistant obligate aerobe, and gram-positive AFB, first isolated from Macacus rhesus monkeys in 1965 (3,8). The primary mode of transmission and the natural habitat of this organism still remains unclear although M. simiae isolates have been recovered from sporadic pseudo-outbreaks from contaminated water supplies (9). Initially, M. simiae was geographically restricted to Southwestern United States, Cuba, Israel and the island of Guadeloupe however rare clinical cases of M. simiae infections including pulmonary, intra-abdominal and disseminated disease in immunocompromised hosts, particularly patients infected with human immunodeficiency virus have now been reported worldwide (3,8,29). It is also a rare cause of cervical lymphadenitis in immunocompetent children and to the best of our knowledge, there have only been two case reports of M. simiae cervical lymphadenitis in children and our case is the first reported case in Australia (3,8,30).
Our case report highlights the diagnostic and therapeutic challenges posed by M. simiae cervical lymphadenitis. Firstly, identification of M. simiae proved difficult. Despite FNA, swabs, and tissue examined by AFB smear, culture and other special stains, standard laboratory techniques including pigmentation, growth rate and biochemical testing were unable to isolate and identify the NTM species (31). It was presumed that the child had MAC infection, based on clinical features, and was thus treated accordingly. Definitive surgical management was initially not favoured because of the concern that an excision would endanger the marginal mandibular branch of her facial nerve.
Choice of antibiotic therapy for M. simiae is difficult as it is the most drug-resistant organism of all the NTM (3,8). In vitro and in vivo, M. simiae has demonstrated resistance to all conventional antituberculous drugs including rifampin and ethambutol and to date, there are no published clinical trials of treatment for M. simiae complex disease due to its rarity (10). In the absence of robust clinical trials, the American Thoracic Society has suggested a clarithromycin-based multiple drug regimen based on several cases that have reported favourable clinical outcomes (8,30). Duration of antimycobacterial therapy is also reportedly variable from 6 months to more than 1 year, based upon clinical response (8,30). Combinations of clarithromycin, ethambutol and ciprofloxacin have been reported to have varying success in adults with disseminated M. simiae infection in the setting of acquired immunodeficiency syndrome (32). In our case, the child received 6 months of clarithromycin-based dual antibiotic therapy followed by 6 months of triple antibiotic-therapy without a significant clinical response. There was no palpable reduction in mass size which was corroborated on repeat imaging, and the child developed intermittent skin breakdown with discharge which necessitated complete excision of the involved nodal mass.
Surgical treatment with complete excision is considered the gold standard treatment of NTM cervical lymphadenitis (4), with incision and curettage an alternative. To date, there is only one randomised controlled trial that compared the cure rates of surgical treatment and conservative antimycobacterial therapy. This study concluded that surgical treatment was the superior of the two with cure rates of 96% compared to 66% (5). Nevertheless, surgical treatments are not without their risks and complications such as wound infection, infection recurrence, formation of sinus tract or haematoma, scarring and poor cosmesis and most importantly, facial palsy from facial nerve damage (1,3,5,8). Arguably, in the absence of clinical trials for conservative antimycobacterial therapy, surgical excision may be the prudent choice of therapy in confirmed cases of M. simiae due to its limited susceptibility to antimicrobial agents (3).
Our reported case, the first of its kind in Australia, was a diagnostic and therapeutic dilemma from onset of symptoms until definitive surgical treatment. While M. simiae is usually associated with immunocompromised individuals and while our patient is immunocompetent, she is of indigenous background, living in foster care and was living rurally at the time of onset of symptoms. There are multiple factors that could explain infection such as contact with contaminated soil or water sources. This case serves to remind treating physicians that atypical and rare mycobacterial infections such as M. simiae should be included in the differential diagnosis of an immunocompetent child with cervical lymphadenitis. If M. simiae is suspected, early surgical intervention should be considered given M. simiae is a multidrug resistant organism that is difficult to cure with antimycobacterial therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-21-50/rc
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-21-50/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-21-50/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parent or legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal on request.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tebruegge M, Pantazidou A, MacGregor D, et al. Nontuberculous Mycobacterial Disease in Children - Epidemiology, Diagnosis & Management at a Tertiary Center. PLoS One 2016;11:e0147513. [Crossref] [PubMed]
- Tebruegge M, Curtis N. Mycobacterium species non-tuberculosis. In: Long S, Pickering L, Prober C. editors. Principles and Practice of Pediatric Infectious Diseases. 4th edition. Philadelphia, US: Saunders/Elsevier, 2012:786-98.
- Patel NC, Minifee PK, Dishop MK, et al. Mycobacterium simiae cervical lymphadenitis. Pediatr Infect Dis J 2007;26:362-3. [Crossref] [PubMed]
- Perdikogianni C, Galanakis E. Non-tuberculous mycobacterial cervical lymphadenitis in the immunocompetent child: diagnostic and treatment approach. Expert Rev Anti Infect Ther 2014;12:959-65. [Crossref] [PubMed]
- Haverkamp MH, Arend SM, Lindeboom JA, et al. Nontuberculous mycobacterial infection in children: a 2-year prospective surveillance study in the Netherlands. Clin Infect Dis 2004;39:450-6. [Crossref] [PubMed]
- Tortoli E. Epidemiology of cervico-facial pediatric lymphadenitis as a result of nontuberculous mycobacteria. Int J Mycobacteriol 2012;1:165-9. [Crossref] [PubMed]
- Zimmermann P, Tebruegge M, Curtis N, et al. The management of non-tuberculous cervicofacial lymphadenitis in children: A systematic review and meta-analysis. J Infect 2015;71:9-18. [Crossref] [PubMed]
- Hankins D, Kelly M, Vijayan V. Mycobacterium simiae Infection of the Parotid Gland in an Immunocompetent Child. J Pediatric Infect Dis Soc 2013;2:394-6. [Crossref] [PubMed]
- El Sahly HM, Septimus E, Soini H, et al. Mycobacterium simiae pseudo-outbreak resulting from a contaminated hospital water supply in Houston, Texas. Clin Infect Dis 2002;35:802-7. [Crossref] [PubMed]
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. [Crossref] [PubMed]
- Daley AJ. Non-tuberculous mycobacterial cervical lymphadenopathy. J Paediatr Child Health 2001;37:78-80. [Crossref] [PubMed]
- Blyth CC, Best EJ, Jones CA, et al. Nontuberculous mycobacterial infection in children: a prospective national study. Pediatr Infect Dis J 2009;28:801-5. [Crossref] [PubMed]
- Joshi W, Davidson PM, Jones PG, et al. Non-tuberculous mycobacterial lymphadenitis in children. Eur J Pediatr 1989;148:751-4. [Crossref] [PubMed]
- Pang SC. Mycobacterial lymphadenitis in Western Australia. Tuber Lung Dis 1992;73:362-7. [Crossref] [PubMed]
- Goutzamanis JJ, Gilbert GL. Mycobacterium ulcerans infection in Australian children: report of eight cases and review. Clin Infect Dis 1995;21:1186-92. [Crossref] [PubMed]
- Wright JE. Non-tuberculous mycobacterial lymphadenitis. Aust N Z J Surg 1996;66:225-8. [Crossref] [PubMed]
- Wark P, Goldberg H, Ferson M, et al. Mycobacterial lymphadenitis in eastern Sydney. Aust N Z J Med 1998;28:453-8. [Crossref] [PubMed]
- Fergusson JA, Simpson E. Surgical treatment of atypical mycobacterial cervicofacial adenitis in children. Aust N Z J Surg 1999;69:426-9. [Crossref] [PubMed]
- O'Brien DP, Currie BJ, Krause VL. Nontuberculous mycobacterial disease in northern Australia: a case series and review of the literature. Clin Infect Dis 2000;31:958-67. [Crossref] [PubMed]
- Flint D, Mahadevan M, Barber C, et al. Cervical lymphadenitis due to non-tuberculous mycobacteria: surgical treatment and review. Int J Pediatr Otorhinolaryngol 2000;53:187-94. [Crossref] [PubMed]
- Sparks R, Khatami A. Mycobacterium fortuitum Complex Skin Infection in a Healthy Adolescent. Infect Disord Drug Targets 2014;14:168-71. [Crossref] [PubMed]
- Chong C, Sinclair J, Voss L, et al. Ophthalmic manifestation of disseminated cutaneous Mycobacterium avium-intracellulare complex in a child with a presumed primary IL-12 receptor defect. Ocul Immunol Inflamm 2015;23:106-9. [Crossref] [PubMed]
- Mahadevan M, Neeff M, Van Der Meer G, et al. Non-tuberculous mycobacterial head and neck infections in children: Analysis of results and complications for various treatment modalities. Int J Pediatr Otorhinolaryngol 2016;82:102-6. [Crossref] [PubMed]
- Freyne B, Curtis N. Mycobacterium gordonae Skin Infection in an Immunocompetent Child. Pediatr Infect Dis J 2017;36:523-5. [Crossref] [PubMed]
- Berkhout A, Curtis N, Gwee A, et al. Mycobacterium abscessus Soft Tissue Infection: Review of Published Cases and Challenges in Treatment. Pediatr Infect Dis J 2020;39:e130-2. [Crossref] [PubMed]
- Aliano D, Thomson R. The Epidemiology of Extrapulmonary Non-tuberculous Mycobacterial Infection in a Pediatric Population. Pediatr Infect Dis J 2020;39:671-7. [Crossref] [PubMed]
- Foley DA, McLeod C, Rodrigues S, et al. A Successful Staged Approach to Management of Mycobacterium fortuitum Cochlear Implant Infection. Pediatr Infect Dis J 2021;40:e47-8. [Crossref] [PubMed]
- Weng A, Curtis N, Kadambari S, et al. Non-tuberculous Mycobacterial Infection of the Retropharyngeal Space. Pediatr Infect Dis J 2022;41:e286-9. [Crossref] [PubMed]
- Valero G, Peters J, Jorgensen JH, et al. Clinical isolates of Mycobacterium simiae in San Antonio, Texas. An 11-yr review. Am J Respir Crit Care Med 1995;152:1555-7. [Crossref] [PubMed]
- Cruz AT, Goytia VK, Starke JR. Mycobacterium simiae complex infection in an immunocompetent child. J Clin Microbiol 2007;45:2745-6. [Crossref] [PubMed]
- Pilkington EF, MacArthur CJ, Beekmann SE, et al. Treatment patterns of pediatric nontuberculous mycobacterial (NTM) cervical lymphadenitis as reported by nationwide surveys of pediatric otolaryngology and infectious disease societies. Int J Pediatr Otorhinolaryngol 2010;74:343-6. [Crossref] [PubMed]
- Al-Abdely HM, Revankar SG, Graybill JR. Disseminated Mycobacterium simiae infection in patients with AIDS. J Infect 2000;41:143-7. [Crossref] [PubMed]
Cite this article as: Doan THL, O’Keeffe J, Agar N, Friedman ND. Mycobacterium simiae: a rare cause of cervical lymphadenitis—case report. Aust J Otolaryngol 2022;5:31.


