
Diagnostic and management considerations in multicentric forms of laryngeal paraganglioma: a systematic review
Introduction
Paragangliomas are rare neoplastic lesions derived from neural crest derivatives and arise from autonomic paraganglion. Head and neck paragangliomas (HNPGLs) typically originate from parasympathetic structures located around the skull base and neck and commonly involve the baroreceptors of the carotid body (60%), glossopharyngeal nerve fibres within the jugulotympanic cavity (40%), or vagal fibres (5%) (1). Rare anatomic locations include the orbit, paranasal sinuses, nasopharynx, parotid, larynx, or thyroid gland. Laryngeal paragangliomas (LPs) comprise a minor subset of HNPGL and arise from either the superior or inferior laryngeal paraganglia, with >90% involving the supraglottis. They are generally non-functional and do not secrete catecholamines, as they are associated with parasympathetic structures. LPs and HNPGLs are usually unifocal and sporadic, with hereditary disease underlying 30–40% of cases (2). Germline mutations of succinate dehydrogenase (SDH) are thought to account for 70% of familial paragangliomas (3). Multicentric forms of HNPGL may be present in 10–20% of sporadic cases and up to 80% of familial cases (4). However, multicentricity in LP has been rarely described in the literature, with reports of synchronous lesions reported in the carotid body, jugular bulb, and skull base (5-15). Paragangliomas presenting with multifocal disease, when compared to solitary lesions, are associated with higher rates of recurrence and patients require stricter surveillance and genetic counselling (16). We present a systematic review of all known cases of LP with multifocal disease and provide a comprehensive overview of the patient’s clinical presentation, diagnostic workup, and management. The objective of the review is to characterise the demographics of patients with multicentric forms of LP, describe the location of synchronous and metachronous lesions found, the imaging modalities incorporated in the diagnostic workup, and the management employed. It is likely that the literature underreports multicentricity as whole-body scanning with octreotide scintigraphy is a recent imaging modality that has not been employed widely until the last decade. We present the following article in accordance with the PRISMA reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-22-11/rc).
Materials and methods
The search strategy aimed to identify all recorded cases of multicentricity in patients with LP in the literature. A literature review was performed on 22nd September 2022 using PubMed, Medline, SCOPUS, and EMBASE databases, with results imported into the reference management software Endnote 20.4 (Clarivate Analytics 2022). Three main search domains were used, which were combined with the Boolean operator “and”, whilst search terms contained within each domain were combined with the Boolean operator “or”. The keywords within the first search domain were “head and neck” or “larynx”, the second search domain was “paraganglioma”, and the third search domain was “multicentric”, “synchronous”, or “metachronous”. Our inclusion criteria included any study that reported a patient with confirmed LP and either (I) biopsy confirmed synchronous lesion, (II) somatostatin-avid synchronous lesion on octreotide PET scan, or (III) suspicious lesion on CT or MRI with distinctive features of paraganglioma (this includes contrast enhancement, internal arterial flow voids, and salt and pepper appearance). All article types within the published literature between 1975 and 2022 were eligible for inclusion. Studies were excluded if they described HNPGLs without larynx involvement. Studies were not excluded based on language and 1 study from the non-English literature was included in the analysis. Google, Google Scholar, and article references were examined to further identify any potentially eligible studies. The search results were reviewed for eligibility by a single reviewer based on the title and abstract of the article, and a full text screening of potentially eligible articles was performed to determine inclusion in the study. The expertise of a consultant head and neck surgeon was available if any uncertainty arose. Articles were either case reports or case series, as previous literature or systematic reviews covering this topic have not been performed. The risk of bias was assessed using the Joanna Briggs Institute checklist, standardised tool developed for case reports and series, and is provided in Figure S1 and Appendix 1 (17).
Demographic data of patients and information regarding the diagnostic workup, paraganglioma, and management was compiled into Microsoft Excel version 16.53 (Microsoft Corporation, Redmond, WA, USA) for Mac (Cupertino, CA, USA). Statistical analysis was performed within the Excel spreadsheet.
Results and outcomes
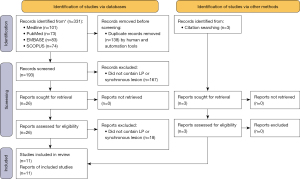
The results of the PRISMA are shown in Figure 1. Eleven articles were included which reported 12 patients who had confirmed LP with a concurrent synchronous biopsy-proven paraganglioma, somatostatin-avid lesion on octreotide scintigraphy, or lesion on CT/MRI with pathognomonic features of paragangliomas. The bias of the included studies was low, with all studies documenting the demographic and clinical characteristics of the patient, the symptomatology of the LP or synchronous lesion, the investigative modalities used, and the type of management for both lesions. One study in a Radiology journal did not mention the management of either the LP or synchronous carotid body tumour. Most studies either did not adequately document follow up or had a short follow up duration.
Demographic data and baseline information of the patient cohort is summarised in Table 1. Almost equal number of male and female patients were recorded, compared to a 3:1 female predilection reported in most studies of LPs (18). The patient cohort was relatively young with a mean age of 46 years, compatible with the literature suggesting that LP usually manifest between the fourth to sixth decades of life (18). Three patients had a confirmed or suspected positive family history of paraganglioma. Dogan described a patient with a synchronous left carotid body paraganglioma (CBP), whose mother had reported an excision of CBP previously (7). Hall reported a case of right supraglottic paraganglioma with a synchronous right CBP, confirmed on histopathology, in a patient who had a brother and 2 sons with glomus tumours (9). The patient had an SDH-B mutation. Schmit reported a patient with left subglottic paraganglioma with a synchronous right skull base lesion demonstrating MRI enhancement, suggestive of a vagal paraganglioma (13). The patient had a grandfather who died from an undiagnosed skull base tumour. The diagnostic workup of LP in our patient cohort included multimodal imaging techniques, with CT and MRI being routine investigations. Whole-body scintigraphy modalities with octreotide-based scans were used in 7 cases. Only 1 LP was functional (i.e., catecholamine secreting), with the overwhelming majority being non-secretory (8).
Table 1
| Study parameters | Patient cohort |
|---|---|
| Age (years) | 46±15 |
| Gender | |
| Male | 5 (41.7) |
| Female | 6 (50.0) |
| Not specified | 1 (8.3) |
| Country | |
| USA | 4 (33.3) |
| India | 2 (16.7) |
| Australia | 2 (16.7) |
| Iran | 1 (8.3) |
| Spain | 1 (8.3) |
| Turkey | 1 (8.3) |
| United Kingdom | 1 (8.3) |
| Positive family history | 3 (25.0) |
| Previous LP | 4 (33.3) |
| Functional LP | 1 (8.3) |
| Imaging modality | |
| Computed tomography | 10 (83.3) |
| Magnetic resonance imaging | 9 (75.0) |
| Octreotide scintigraphy (e.g., 68-gallium DOTATATE) | 7 (58.3) |
| Angiogram (CT or MRI) | 5 (41.7) |
| Ultrasound | 3 (25.0) |
Values are expressed as n (%), to 1 significant figure, or mean ± standard deviation where appropriate. LP, laryngeal paraganglioma; CT, computed tomography; MRI, magnetic resonance imaging.
LP characteristics
Clinical information regarding the characteristics of the identified LP is summarised in Table 2. The overwhelming majority of LP was supraglottic, with only 1 case of subglottic paraganglioma arising from the inferior laryngeal paraganglia reported. The main symptoms reported in the literature include neck mass or swelling (n=6), dysphonia (n=5), dyspnea (n=3), dysphagia (n=1), stridor (n=1), and haemoptysis (n=1). The symptoms of neck swelling or palpable neck lump could also be attributable to the synchronous paraganglioma, as 5 of the patients had a carotid body tumour. Four patients had a history of previous LP, with the current presentation being a recurrence. Multicentric disease likely has an underlying genetic component and is associated with higher risk of recurrence and treatment failure. Hall reported a patient with SDH-B mutation who had recurrent LP after a previous surgical excision 15 years previously (9). Sankar reported a patient who had endoscopic resection of LP in 2003, 2006, and 2014, and represented in 2017 with supraglottic paraganglioma with multicentric disease present in the cervical lymph nodes and multiple cutaneous nodules of the upper back, abdomen, and pelvis (12). Octreotide scintigraphy showed concern for metastatic disease with avid lesions in the liver. Sharifkashany described a patient who had a recurrence 5 years after suprahyoid incision and resection of LP (14). We have also described a case of LP in a female patient who presented with a left supraglottic LP on a background of previous bilateral carotid body tumours (CBTs) that were managed with angioembolisation and surgical resection.
Table 2
| Study | Symptoms | Duration (months) | Imaging modalities | LP characteristics | Management |
|---|---|---|---|---|---|
| Abt, 2020 | Neck mass | N/A | CT, MRI, octreotide | 17 mm left supraglottic mass involving AE fold | External cervical approach |
| Ananthapadmanabhan, 2022 | Dysphonia, dyspnea, dysphagia | 24 | CT, MRI, octreotide | 21 mm left supraglottic mass involving AE fold and false vocal cord | External cervical approach |
| Dyspnea | N/A | CT, octreotide | Left supraglottic mass involving false vocal cord | External cervical approach | |
| Dogan, 2015 | Neck mass | 18 | CT, MRI, octreotide, USS, angiogram | 10 mm × 12 mm right supraglottic mass involving pre-epiglottic space; DSA: STA feeding vessel | Angioembolisation STA; external cervical approach |
| García-Martín, 2010 | Dysphonia, dyspnea | 36 | CT, MRI, octreotide, angiogram | 32 mm × 20 mm × 22 mm right supraglottic mass involving AE fold | Angioembolisation; external cervical approach |
| Hall, 2010 | Neck mass | 12 | CT, MRI, USS, angiogram | 16 mm × 12 mm right glottic mass involving true vocal cord, extending into subglottis | Angioembolisation STA; external cervical approach |
| Rubin, 2005 | Dysphonia, neck mass, stridor | 24 | CT, MRI, angiogram | Left supraglottic mass involving AE fold with 50% airway obstruction; angiogram: STA feeding vessel | Angioembolisation STA; external cervical approach |
| Sanders, 2001 | Haemoptysis | N/A | CT, MRI, angiogram | Left supraglottic mass; angiogram: SLA feeding vessel | Angioembolisation STA; external cervical approach; supraglottic laryngectomy |
| Sankar, 2018 | Dysphonia | N/A | CT, octreotide (technetium-99m scan) | Supraglottic mass involving bilateral AE folds | Previous endoscopic resection ×3; chemotherapy for current recurrent presentation |
| Schmit, 2006 | Neck mass | N/A | MRI, USS | 30 mm × 30 mm × 25 mm left subglottic mass | External cervical approach |
| Sharifkashany, 2014 | Dysphonia | 60 | CT, MRI | 20 mm × 15 mm left supraglottic mass involving AE fold and pre-epiglottic space | Previous external cervical approach; radiotherapy for current recurrent presentation |
| Tripathy, 2017 | Neck mass | 6 | CT, octreotide | 8 mm × 10 mm left supraglottic mass involving false vocal cord | N/A |
Majority of patients underwent external cervical approach for surgical resection of the lesion. Five patients underwent pre-operative angioembolisation of the superior thyroid artery, which was identified on angiogram as the feeding artery to the lesion. LP, laryngeal paraganglioma; N/A, not applicable; CT, computed tomography; MRI, magnetic resonance imaging; AE, aryepiglottic; USS, ultrasound scan; DSA, digital subtraction angiography; STA, superior thyroid artery; SLA, superior laryngeal artery.
Synchronous paraganglioma characteristics
Eighteen synchronous lesions were described in the literature in the 12 patients, and summarised in Table 3. This includes 10 CBPs in 7 patients, with 3 having bilateral carotid body involvement. Other foci of involvement include the skull base (n=4) and parapharyngeal space, which could represent carotid body or vagal paragangliomas. Rubin described a patient with supraglottic paraganglioma with concurrent disease involvement of the left common carotid bifurcation, the right carotid body, and a small lesion in the petrous temporal bone (10). The skull base lesion likely represented another CBP affecting the proximal portion of the petrous segment of the internal carotid artery (ICA). Schmit reported a synchronous MRI enhancing 1.5 cm lesion located 2 cm below the right skull base posterior to the right ICA, likely representing a vagal paraganglioma near the jugular foramen (13). Sharifkashany reported a patient with recurrent supraglottic paraganglioma with 2 synchronous contrast-enhancing lesions on CT and MRI (14). An expansile-lytic left jugular foramen mass, representing a biopsy-confirmed jugulotympanic paraganglioma (or glomus jugulare or tympanicum), demonstrated erosion of the skull base including the petrous apex and the bony septum between the jugular foramen and carotid canal. The mass extended into the middle ear cleft causing obstruction of the mastoid antrum with air cell opacification. A second synchronous lesion was observed in the right parapharyngeal space, involving the right pharyngeal mucosal space of the nasopharynx—the absence of splaying of the carotid bifurcation and posterior displacement of the ICA is suggestive of a vagal, rather than a carotid body, paraganglioma (19). Finally, the authors have previously described a case of supraglottic paraganglioma with a left para-aortic paraganglioma, as an incidental finding on octreotide scan, representing the first confirmed synchronous lesion in patients with LP outside the head and neck (Figure 2) (6). In this paper, we also reported a case with synchronous octreotide-avid lesions in the bilateral skull base and left paravertebral space.
Table 3
| Study | Symptoms | Synchronous paraganglioma characteristics | Management | Genetic mutation |
|---|---|---|---|---|
| Abt, 2020 | Neck mass | 28 mm left CBP, 17 mm right CBP | Staged resection via external cervical approach | SDH-B |
| Ananthapadmanabhan, 2022 | Nil | 21 mm left para-aortic mass | Laparoscopic resection | Dual SDH-A, SDH-B |
| Nil | Octreotide avid lesions at right and left skull base, left paravertebral region at C2 | Monitoring with serial octreotide scans; previous bilateral CBTs 15 years ago managed with angioembolisation of STA and surgical resection with external cervical approach | N/A | |
| Dogan, 2015 | Neck mass | 25 mm × 32 mm left carotid bifurcation mass; angiogram: left ascending pharyngeal artery, feeding vessel | Angioembolisation of left ascending pharyngeal artery; external cervical approach for resection | N/A |
| García-Martín, 2010 | Nil | 22 mm × 14 mm × 12 mm right CBP | Angioembolisation; external cervical approach for resection | N/A |
| Hall, 2010 | Neck mass | Right CBP; right level V cervical lymph node | Angioembolisation; external cervical approach for resection | SDH-B |
| Rubin, 2005 | Neck mass, right otalgia | 30 mm × 20 mm left CBP, small right CBP, petrous temporal bone lesion | Angioembolisation and resection of left CBP; monitoring of right CBP and skull base lesion | N/A |
| Sanders, 2001 | Nil | 40 mm left CBP | External cervical approach for resection | N/A |
| Sankar, 2018 | Multiple painful cutaneous nodules | Multiple cutaneous nodules on back, abdomen, pelvis; segment V, VIII liver; cervical lymph nodes | Chemotherapy | N/A |
| Schmit, 2006 | Nil | 15 mm right skull base, likely vagal paraganglioma | Monitoring via imaging | N/A |
| Sharifkashany, 2014 | Left otalgia, CN VII palsy, mixed hearing loss | 35 mm × 25 mm right parapharyngeal space lesion; 30 mm × 25 mm left jugulo-tympanic mass | Radiotherapy | N/A |
| Tripathy, 2017 | Neck mass | 24 mm × 16 mm × 30 mm right CBP; 40 mm × 35 mm × 52 mm left CBP | N/A | N/A |
CBP, carotid body paraganglioma; SDH, succinate dehydrogenase; CBTs, carotid body tumours; N/A, not applicable; CN, cranial nerve.
Management
Surgical management of LP via an external cervical approach is associated with less chance of recurrence compared to an endoscopic or microlaryngeal approach. This was the preferred treatment modality in 9 patients, with 5 who also had pre-operative angioembolisation of the feeding vessel, the superior thyroid artery. The patient in Sharifkashany was referred for radiotherapy for recurrent disease with multicentric lesions occurring in areas with difficult surgical access (14). Sankar et al. referred their patient for chemotherapy for metastatic and malignant disease involving cervical lymph nodes, cutaneous nodules, and suspicious hepatic nodules (12). There were additional management considerations for synchronous HNPGLs, especially in vagal, tympanic, and skull base lesions where surgical access is complicated compared to CBP and LPs. The size, symptom profile, and bilaterality of lesions affected decision to resect versus monitor the disease. Post-management follow-up was reported in only 7 studies with a mean duration of 5.1±2.7 months, with no study reporting residual or recurrent disease. Genetic testing was only performed in 3 studies, all confirming SDH-B mutation, with 1 patient having combined SDH-A and SDH-B germline mutation (5,6,9).
Discussion
Genetic considerations
Multicentric forms of disease in paragangliomas has prognostic implications. They are more likely to have an underlying hereditary component occurring in up to 80% of familial paragangliomas compared to 10–20% of sporadic paragangliomas (4). Germline loss-of-function mutations in SDH are recognised as susceptibility genes for familial paragangliomas. These genes encode mitochondrial complex proteins and are transmitted in an autosomal dominant pattern with incomplete penetrance. Other genes of interest include hypoxia-inducible factor (HIF)-2 and von Hippel-Lindau (VHL). These genes coordinate angiogenesis and cellular proliferation in hypoxia, and mutations can lead to inappropriate activation of hypoxia pathways. It is postulated that the oxygen-sensing capabilities of paraganglionic tissues including the carotid body and glossopharyngeal and vagal fibres are defective, leading to chronic hypoxic stimulation, cell proliferation, and tumorigenesis (20). A retrospective review of 214 patients with HNPGL, of which 47 had confirmed genetic mutation of SDH, showed that mutation-positive patients had higher incidence of bilateral, functional, malignant, and metachronous tumours, as well as higher rates of recurrence and treatment failure (16). Papaspyrou et al. studied 175 patients with 225 HNPGLs, in which 19% of patients had multicentric disease—the incidence in mutation-positive patients was 65% (21). In our systematic review, 4 patients presented with recurrent LP. However, the short mean follow-up period in the included studies was a limitation as we cannot satisfactorily determine whether there was oncologic clearance or disease recurrence post intervention—in most studies, recurrence manifests several months or years after resection. The importance of detecting multicentric disease is manyfold. It stratifies patients into high-risk groups that require more stricter and regular surveillance, and allows us to refer patients for targeted genetic workup and appropriate counselling, as family members may need screening for hereditary paragangliomas. Hereditary disease is also likely underreported—only 3 patients with multicentric LP in our systematic review underwent genetic testing and all had SDH-B mutations. It is probable that genetic mutations were implicated in some of the other patients as well.
Clinical manifestations of LPs and synchronous lesions
In our systematic review it is important to note that LPs are more likely, compared to synchronous lesions, to become symptomatic as they readily affect voicing, swallowing, or breathing. Supraglottic lesions can create mass effect that can affect the oscillatory function of the vocal cords, manifesting as dysphonia, and create a mechanical obstruction to food bolus transit, causing dysphagia or aspiration. Airway obstruction from lesions overlying the glottis can manifest with exertional, or in severe cases, resting, dyspnea and inspiratory stridor. Synchronous HNPGL presented with neck masses if significant in size. Otherwise, symptoms only arose if the paraganglioma caused compression of anatomical structures or destruction of normal anatomy—1 notable case was the patient with facial palsy and mixed hearing loss secondary to an expansile jugulotympanic paraganglioma that caused erosion of the petrous temporal bone and invasion into the middle ear space (14). This is important to consider in the diagnostic workup of the suspected LP—synchronous lesions outside the head and neck region are likely to be asymptomatic, unless secretory, and will be missed if imaging is restricted to the head and neck. Nevertheless, most synchronous lesions in HNPGL also occur in the head and neck. This may be because the underlying mutation affects oxygen-sensing structures that are restricted to the head and neck.
Role of whole-body scanning in detection of multicentric disease
Octreotide scintigraphy with 68-gallium labelled somatostatin scanning is emerging as a useful imaging modality in the standard diagnostic workup of a patient with suspected paraganglioma. Whereas conventional cross-sectional imaging techniques including CT and MRI are limited to a region of interest, octreotide-based scans screen the whole body for somatostatin-receptor expression to diagnose synchronous, metachronous, and metastatic disease. The increasing incidence of multicentric LP in recent literature likely coincides with the utilisation of whole-body screening techniques. In our systematic review, all 3 studies prior to 2010 did not utilise scintigraphy, whilst 6 of the 8 studies during or after 2010 incorporated octreotide based scanning techniques (5-15). Previous studies in patients with HNPGL showed that 68-gallium octreotide scans demonstrate superiority over conventional CT/MRI neck in detecting smaller lesions (22-24). The higher disease sensitivity and diagnostic accuracy is attributable to the improved spatial resolution. Naswa et al. showed that incorporating 68-gallium octreotide scanning altered management in 3 out of 5 patients with HNPGL by detecting synchronous or metastatic disease (25). The intent or modality of treatment in these patients were affected, with some requiring palliative rather than curative management and some requiring radiotherapy. Furthermore, patients with concurrent disease outside the head and neck region are less likely to be symptomatic unless the lesion is secretory or large enough to cause a mass effect. Hence, synchronous disease would be missed if whole-body scintigraphy is omitted in the baseline evaluation. It is likely that older literature underestimated the prevalence of multicentric disease in both HNPGL and LP. Some authors have hypothesised that undiagnosed synchronous lesions may act as a nidus for recurrence (15), though this may also be driven by an underlying genetic mutation.
Management considerations in multicentric paragangliomas
Surgical resection of paragangliomas remains the only curative treatment modality for LPs. The management of unifocal LP is straightforward and not controversial—the lesions are amenable to excision via an external cervical approach, with complete resection of the lesion and preservation of laryngeal structures. An open approach is favourable to an endoscopic approach, by providing good surgical access to and visualisation of the lesion, easy vascular control, and lower rates of treatment failure. This achieves good tumour clearance without the need for adjuvant treatment modalities. However, in the patient with multicentric disease, surgical planning must take into account multiple factors including the size and anatomic location of the synchronous lesions, unilaterality versus bilaterality, association with cranial nerve deficits, and if they are symptomatic or asymptomatic (3,5,26). Whereas laryngeal and carotid body tumours can be easily accessed by an external cervical approach, the surgical access to skull base paragangliomas, including jugulotympanic and vagal lesions, is complex and high-risk for injury to vascular and cranial nerve structures. A retrospective review of 24 cases of multicentric HNPGL at a single institution showed high incidence of post-operative cranial nerve deficits, affecting the facial (19.2%), glossopharyngeal (7.7%), vagus (30.7%), spinal accessory (11.5%), and hypoglossal (7.7%) nerves (3). Hence, the surgeon must decide whether surgical excision is feasible and safe, consider both patient and tumour factors, and if alternate modalities should be considered including stereotactic radiotherapy or careful monitoring with serial imaging.
Bilaterality is commonly seen in patients with CBP, including 4 patients in our systematic review, and is associated with multicentric and hereditary disease. The literature suggests that bilateral HNPGL is present in 4.4% of sporadic disease and 31.8% of familial disease (27). A staged approach to surgical excision is required in bilateral disease to minimise the risk of iatrogenic injury to bilateral lower cranial nerves, which causes significant disability in terms of voice and swallow outcomes (3,26). Current literature recommends excision of the synchronous lesion on the same side of the LP first to avoid bilateral neuropraxia. In cases with bilateral carotid or vagal paraganglioma, if there is a large tumour it is recommended to excise the smaller lesion first. An interval of 6 months between procedures is advised to allow the initial carotid body to heal in order to avoid baroreceptor reflex syndrome, a condition of secondary hypertension due to bilateral denervation of the carotid body baroreceptors (3,28). In patients with CBP and other HNPGL on the contralateral side, the carotid body tumour should be excised first as the easy surgical access via a transcervical approach is associated with the least risk of lower cranial nerve damage (3). In patients with synchronous HNPGL who present with cranial nerve deficits, it is advised to excise the lesion on the side of the palsy first. In our systematic review, the patients with LP and a synchronous skull base paraganglioma, all had non-surgical management of the synchronous lesion. Skull base tumours can be accessed by an infratemporal, transmastoid, or transcondylar approach, with risk of injuring lower cranial nerves, especially the vagus. Observation with serial imaging should be considered in elderly patients, poor surgical candidates, small asymptomatic lesions, patients with bilateral or recurrent disease, and patients with a post-operative cranial nerve palsy after excision of a previous paraganglioma. This is reasonable as the overwhelming majority of paragangliomas are slow growing. Large lesions with concern for osseous destruction of the skull base and intradural invasion should be considered for surgical intervention in ideal candidates. It is important to recognise that the size of the tumour is directly proportional to the risk of post-operative cranial nerve deficits (3). Pre-operative selective angioembolisation of the feeding vessel can assist in shrinking the second lesion prior to resection to minimise accidental nerve trauma. In skull base paragangliomas, stereotactic radiotherapy has demonstrated adequate tumour control with lower morbidity compared to surgery (29,30). It should be considered in cases with difficult access, in patients presenting with cranial nerve deficits, and in bilateral multicentric disease. Radiotherapy is not curative and only halts tumour growth. Patient preference is another important factor in deciding management.
Detecting recurrent and metastatic lesions has important implications in surgical planning, as patients may require adjuvant treatment modalities such as stereotactic radiotherapy or chemotherapy. Malignant or metastatic disease has no specific histopathological features and lacks strict definition within the literature—it could be considered as lymph node involvement, destructive or erosive lesions, or distant metastases. The existence of metastatic or malignant LPs are controversial in the literature, with some experts in the field believing may represent misdiagnosed atypical carcinoid tumours (31,32). The presence of widespread or destructive paragangliomas is more common in multicentric or hereditary disease and may require palliative treatment modalities if surgical resection and tumour clearance is unfeasible.
Limitations
Limitations in this systematic review warrant discussion. The rarity of LPs, and to a greater extent the co-existence with synchronous disease, is reflected in the paucity of the literature. The evidence largely stems from case reports and case series, and hence the quality of the evidence must be taken into account. Even the literature on HNPGLs in general is limited, and mostly focussed on carotid body tumours with some representation from glomus vagale, tympanicum, or jugulare. The evidence from the HNPGL literature was used to guide the discussion on diagnosis and management in our review of multicentric LPs, as the majority of synchronous disease has been historically described in the head and neck region.
The bias of the included studies was low (see Figure S1 and Appendix 1) as the main data that was extracted included whether there was a diagnosis of LP and synchronous disease and how each were managed. As there is no quantitative data or effect estimate being measured, publication bias cannot be measured. Possible limitations in the included studies arose in heterogeneity in post-operative follow-up, with most studies reporting either no follow up or a short duration—this would likely underestimate the incidence of disease recurrence and treatment failure. Different studies also do not report the exact same type of data, which can limit comparisons being made. Finally, the single-author screening in the systematic review is a potential limitation—however given the data extraction was whether the included studies reported a LP with multifocal disease or not, it is unlikely to have been affected by significant bias. If uncertainty arose, it was escalated to a consultant head and neck surgeon for expert opinion.
This study represents the first review article on LP with synchronous or multicentric disease and can provide otolaryngologists and clinicians with important information on how to approach and manage this rare condition.
Conclusions
Multicentricity accounts for a minor subset of LPs but has clinically significant impact on surgical management and prognosis. They are associated with increased risk of recurrence and familial disease, and mandate stricter surveillance and consideration for genetic workup. The incidence of multicentric disease in LPs is likely underestimated and incorporating whole-body octreotide scanning can improve detection of synchronous and metachronous lesions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-22-11/rc
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-22-11/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-22-11/coif). FR serves as an unpaid editorial board member of Australian Journal of Otolaryngology from January 2019 to December 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pellitteri PK, Rinaldo A, Myssiorek D, et al. Paragangliomas of the head and neck. Oral Oncol 2004;40:563-75. [Crossref] [PubMed]
- Williams MD. Paragangliomas of the Head and Neck: An Overview from Diagnosis to Genetics. Head Neck Pathol 2017;11:278-87. [Crossref] [PubMed]
- Álvarez-Morujo RJ, Ruiz MÁ, Serafini DP, et al. Management of multicentric paragangliomas: Review of 24 patients with 60 tumors. Head Neck 2016;38:267-76. [Crossref] [PubMed]
- Lee JH, Barich F, Karnell LH, et al. National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer 2002;94:730-7. [Crossref] [PubMed]
- Abt NB, Holcomb AJ, Shroff S, et al. Intralaryngeal paraganglioma workup and discussion of surgical approach. BMJ Case Rep 2020;13:234745. [Crossref] [PubMed]
- Ananthapadmanabhan S, Jabbour J, Tseros E, et al. Operative technique in laryngeal paraganglioma: A case series and literature review. Laryngoscope Investig Otolaryngol 2022;7:467-75. [Crossref] [PubMed]
- Dogan S, Senol S, Imamoglu H, et al. An Unusual Case of Laryngeal Paraganglioma in a Patient with Carotid Body Paraganglioma: Multimodality Imaging Findings. Case Rep Radiol 2015;2015:342312. [Crossref] [PubMed]
- García-Martín A, Cortés-Berdonces M, Muros MA, et al. Dysphonia as first and single symptom of multicenter paraganglioma. Rev Clin Esp 2010;210:e29-30. [PubMed]
- Hall TC, Renwick P, Stafford ND. Recurrent familial malignant carotid body tumour presenting with lymph node metastasis: case report, and review of diagnosis and management of familial carotid body tumours. J Laryngol Otol 2010;124:1344-6. [Crossref] [PubMed]
- Rubin AD, Cheng SS, Bradford CR. Laryngeal paraganglioma in a patient with multiple head and neck paragangliomas. Otolaryngol Head Neck Surg 2005;132:520-2. [Crossref] [PubMed]
- Sanders KW, Abreo F, Rivera E, et al. A diagnostic and therapeutic approach to paragangliomas of the larynx. Arch Otolaryngol Head Neck Surg 2001;127:565-9. [Crossref] [PubMed]
- Sankar G, Rajaraman V, Ganesh RN, et al. Multiple Cutaneous Metastases on 99mTC-HYNIC-TOC Scan in a Rare Case of Malignant Laryngeal Paraganglioma. Indian J Nucl Med 2018;33:348-50. [Crossref] [PubMed]
- Schmit GD, Gorman B, van Heerden JA, et al. Inferior laryngeal paraganglioma mimicking a primary thyroid tumor. Endocr Pract 2006;12:432-5. [Crossref] [PubMed]
- Sharifkashany S, Yazdani N, Ghazavi H, et al. Laryngeal paraganglioma: a case report. Iran J Radiol 2014;11:e21011. [Crossref] [PubMed]
- Tripathy S, Mukherjee A, Singh CA, et al. Glomus Tumor of the Larynx: A Rare Synchronous Paraganglioma in a Patient with Bilateral Carotid Body Tumor Detected on 68Ga-DOTANOC PET/CT. Indian J Nucl Med 2017;32:241-2. [Crossref] [PubMed]
- Sen I, Young WF Jr, Kasperbauer JL, et al. Tumor-specific prognosis of mutation-positive patients with head and neck paragangliomas. J Vasc Surg 2020;71:1602-12.e2. [Crossref] [PubMed]
- Joanna Briggs Institute. Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools
- Ferlito A, Devaney KO, Rinaldo A. Neuroendocrine neoplasms of the larynx: advances in identification, understanding, and management. Oral Oncol 2006;42:770-88. [Crossref] [PubMed]
- Wang X, Chen Y, Chen X, et al. Parapharyngeal space paraganglioma: distinguishing vagal paragangliomas from carotid body tumours using standard MRI. Clin Radiol 2019;74:734.e1-6. [Crossref] [PubMed]
- Baysal BE. Genetics of familial paragangliomas: past, present, and future. Otolaryngol Clin North Am 2001;34:863-79. vi. [Crossref] [PubMed]
- Papaspyrou K, Mewes T, Rossmann H, et al. Head and neck paragangliomas: Report of 175 patients (1989-2010). Head Neck 2012;34:632-7. [Crossref] [PubMed]
- Tan TH, Hussein Z, Saad FF, et al. Diagnostic Performance of (68)Ga-DOTATATE PET/CT, (18)F-FDG PET/CT and (131)I-MIBG Scintigraphy in Mapping Metastatic Pheochromocytoma and Paraganglioma. Nucl Med Mol Imaging 2015;49:143-51. [Crossref] [PubMed]
- Jaiswal SK, Sarathi V, Malhotra G, et al. The utility of 68Ga-DOTATATE PET/CT in localizing primary/metastatic pheochromocytoma and paraganglioma in children and adolescents - a single-center experience. J Pediatr Endocrinol Metab 2020;34:109-19. [Crossref] [PubMed]
- Sharma P, Thakar A, Suman K C S, et al. 68Ga-DOTANOC PET/CT for baseline evaluation of patients with head and neck paraganglioma. J Nucl Med 2013;54:841-7. [Crossref] [PubMed]
- Naswa N, Kumar A, Sharma P, et al. Imaging carotid body chemodectomas with 68Ga-DOTA-NOC PET-CT. Br J Radiol 2012;85:1140-5. [Crossref] [PubMed]
- Szymańska A, Szymański M, Czekajska-Chehab E, et al. Diagnosis and management of multiple paragangliomas of the head and neck. Eur Arch Otorhinolaryngol 2015;272:1991-9. [Crossref] [PubMed]
- Myssiorek D, Ferlito A, Silver CE, et al. Screening for familial paragangliomas. Oral Oncol 2008;44:532-7. [Crossref] [PubMed]
- De Toma G, Nicolanti V, Plocco M, et al. Baroreflex failure syndrome after bilateral excision of carotid body tumors: an underestimated problem. J Vasc Surg 2000;31:806-10. [Crossref] [PubMed]
- Hinerman RW, Mendenhall WM, Amdur RJ, et al. Definitive radiotherapy in the management of chemodectomas arising in the temporal bone, carotid body, and glomus vagale. Head Neck 2001;23:363-71. [Crossref] [PubMed]
- Foote RL, Pollock BE, Gorman DA, et al. Glomus jugulare tumor: tumor control and complications after stereotactic radiosurgery. Head Neck 2002;24:332-8; discussion 338-9. [Crossref] [PubMed]
- Myssiorek D, Rinaldo A, Barnes L, et al. Laryngeal paraganglioma: an updated critical review. Acta Otolaryngol 2004;124:995-9. [Crossref] [PubMed]
- Suárez C, Rodrigo JP, Ferlito A, et al. Tumours of familial origin in the head and neck. Oral Oncol 2006;42:965-78. [Crossref] [PubMed]
Cite this article as: Ananthapadmanabhan S, Jabbour J, Suruliraj A, Riffat F, Smith M, Sritharan N. Diagnostic and management considerations in multicentric forms of laryngeal paraganglioma: a systematic review. Aust J Otolaryngol 2022;5:30.


