Midline posterior glossectomy and lingual tonsillectomy in children with refractory obstructive sleep apnoea: factors that influence outcomes
Introduction
Paediatric obstructive sleep apnoea (OSA) is seen in 1–3% of children, particularly in the pre-school years (1). Long term, it can lead to failure to thrive, pulmonary hypertension, and learning and behavioral difficulties (2). Adenotonsillectomy (AT) is the first-line therapy for children with OSA and the first-stage treatment for complex OSA patients with evidence of adenotonsillar hypertrophy. In otherwise healthy children, 80% will improve clinically and normalize their polysomnogram (PSG) after AT (1,3). However, those who fail first-line therapy may require additional intervention such as positive airway pressure (PAP) therapy and further surgery.
Persistent or refractory OSA (rOSA) in children can be defined as airway obstruction on PSG despite first-line therapy (4). It is a difficult condition to treat due to its multi-modal aetiology and requires a multidisciplinary approach to improve patient outcomes. Multiple studies have shown when rOSA is associated with obesity, asthma, craniofacial abnormalities and neuromuscular diseases, the rate of complete resolution of OSA after AT drops to 25–45% (2,4-11).
Furthermore, syndromic patients are likely to have multi-level obstruction, including nasoseptal obstruction, macroglossia, retrognathia with glossoptosis, lingual tonsil hypertrophy, lateral pharyngeal collapse, laryngomalacia and vocal cord paralysis (2). Clinically, the causative level and degree of obstruction can be assessed using a combination of history and examination, drug induced sleep endoscopy (DISE) and Cine magnetic resonance imaging (MRI) (12). Therefore, surgical options can be tailored to the level of obstruction, which may include turbinoplasty, tongue base reduction (TBR), supraglottoplasty or tracheostomy.
The current gold standard management for paediatric rOSA is PAP therapy (1,2). However, its tolerance in syndromic children is poor and long-term adherence is made more difficult by the need for frequent refitting of masks due to the rapid growth of children (13,14). This, in conjunction with evidence supporting the use of Cine MRI and DISE, has resulted in an increase in individualized, resistance-based surgical management (12).
TBR can be performed by lingual tonsillectomy (LT) or midline posterior glossectomy (MPG) or a combination of these procedures. LT treats hypertrophied lymphoid tissue on the surface of the posterior one-third of the tongue and is relatively common. MPG involves the partial excision of lingual musculature for patients with glossoptosis or macroglossia, where LT alone is unlikely to be successful. MPG is a more complicated procedure and not universally performed. As such, there is a paucity of data regarding the outcomes of this procedure.
International studies have assessed the benefit of TBR in rOSA with LT and MPG (15-26).
LT
Lin and Koltai first described endoscopic-assisted CoblationTM LT in 2009 and found a trend towards reduced mean obstructive apnoea hypopnoea index (OAHI) from 14.7 to 8.1 in children with rOSA (15). Abdel-Aziz et al. conducted a retrospective study of 16 children with rOSA who underwent LT and observed an improvement on post-operative PSG in all patients, but persistence of snoring in 37.5% of patients (16). DeMarcantonio et al. demonstrated a statistically significant reduction in median apnoea-hypopnoea index (AHI) and OAHI, and improved median oxygen saturation in 18 patients who had pre- and postoperative PSG (17). Two studies examined outcomes of LT in DS children: Skirko et al. found no appreciable change in mean OAHI, whereas Prosser et al. found significant improvements in AHI, OAHI and oxygen saturation (18,19).
MPG
Only a handful of studies have examined the outcomes of MPG in paediatric rOSA. Propst et al. conducted a retrospective study of 13 children with down syndrome (DS) and rOSA who underwent MPG with or without LT, where they found a statistically significant reduction in mean OAHI in normal-weight children but not in obese children (20). Wootten et al. conducted a retrospective study of 31 consecutive children with rOSA, of which 16 underwent MPG (21). They found a statistically significant reduction in OAHI and improved symptomatology. However, results were confounded by multiple other concurrent procedures, and it was difficult to isolate the outcomes of MPG alone. More recently, Ulualp conducted a retrospective study of 10 children who underwent MPG and LT and found a statistically significant reduction in OAHI in all children, with resolution of apneic events in children with normal weight (22). To date only 52 cases of MPG in children have been reported in the literature (20-24).
Two meta-analyses on TBR in paediatric rOSA were published in 2017. One evaluated the efficacy of LT only, which included 73 patients from 4 studies (25). The overall success rate of LT was 17% for a postoperative AHI less than 1 and 51% for a postoperative AHI less than 5. This study also identified the lack of evidence regarding factors influencing surgical outcome. Another meta-analysis included 11 studies with a total of 114 patients, of which only 24 had MPG (26). AHI improved by 48.5%. There was a greater improvement in AHI in non-syndromic children (59.2% OAHI reduction) compared to syndromic children (40.0% OAHI reduction). Children with DS had less improvement compared to children with other syndromes, but this finding was confounded by the increased mean body mass index (BMI) in the DS group (27 kg/m2 compared to 18 kg/m2).
The aim of the present study is to determine the efficacy of MPG and/or LT in the treatment of paediatric rOSA and to identify the factors that influence surgical outcome. We present the following article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-21-35/rc).
Methods
A retrospective study of all consecutive TBR cases was conducted at a single tertiary paediatric centre (Perth Children’s Hospital) in Western Australia. Data was obtained from review of an electronic operating theatre database and patient case files from 1st January 2007 to 30th June 2021. Inclusion criteria were: (I) paediatric patients aged ≤16 years, (II) patients who underwent TBR by MPG and/or LT and (III) patients with OSA who had failed AT and/or PAP therapy. Patients who underwent TBR for a primary indication other than OSA were excluded. Patients who underwent TBR with concurrent AT, revision adenoidectomy, cautery of inferior turbinates and turbinoplasty were included in this study.
Data on patient demographics, comorbidities, surgical procedures, and pre- and postoperative PSG results were collected.
Polysomnography
PSG was conducted according to the Australasian Sleep Association/Australasian Sleep Technologists’ Association (ASA/ASTA) Paediatric Working Party’s Guidelines for Recording and Scoring of Paediatric Sleep, which is based on the American Academy of Sleep Medicine (AASM) 2007 manual (27,28). All patients were scored according to the paediatric OSA severity criteria (Table 1).
Table 1
| Severity | OAHI |
|---|---|
| Normal | OAHI ≤1/hr TST |
| Mild OSA | OAHI >1/hr to ≤5/hr TST |
| Moderate OSA | OAHI >5/hr to ≤10/hr TST |
| Severe OSA | OAHI >10/hr TST |
†, according to the ASA/ASTA Paediatric Working Party’s Guidelines for Recording and Scoring of Paediatric Sleep, which is based on the AASM 2007 manual; ‡, paediatric OSA severity criteria. OSA, obstructive sleep apnoea; OAHI, obstructive apnoea hypopnoea index; TST, total sleep time; ASA, Australasian Sleep Association; ASTA, Australasian Sleep Technologists’ Association; AASM, American Academy of Sleep Medicine.
Surgical technique
All procedures were performed under general anaesthesia by a single consultant otolaryngologist (senior author). All patients received a prophylactic dose of intravenous dexamethasone (0.25 mg/kg) on induction. Patients were positioned supine with shoulder roll and head ring. MPG was performed with the patient suspended with a laryngoscope in situ and using a Hopkins rod lens telescope and video camera to visualize the epiglottis and central tongue base. The CoblationTM Evac 70 Xtra or the Procise EZ Wand (ArthroCare Corp., Smith and Nephew, Sunnyvale, CA, USA) were used depending on surgical access. The central portion of the tongue base was resected up to the width of the epiglottis to avoid injury to the lingual arteries and deep to the point where the hyoid bone was palpable but not exposed. LT was performed with a Boyle-Davis mouth gag or suspension laryngoscope to expose the lingual tonsils. The tissue was then ablated using the CoblationTM wands.
Postoperatively, most patients were extubated in theatre and observed for at least 24 hours in the intensive care unit (ICU). Analgesia was managed with a combination of paracetamol and ibuprofen, and oxycodone or tramadol as needed.
Outcome measures
The primary outcome of the study was postoperative resolution of rOSA, defined by postoperative OAHI <5, ≥50% reduction in preoperative OAHI or symptomatic improvement with complete cessation of PAP therapy.
Secondary analyses were performed to investigate whether gender, BMI, and the presence of a syndromic diagnosis influenced the primary outcome.
Statistical analysis
Data was analysed using IBM SPSS Statistics for Windows, version 20 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to summarize the study population. Comparison of pre- and postoperative PSG results and secondary analyses were performed using paired sample t-tests. P value <0.05 was considered statistically significant.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the local research ethics committee (approval number: 26695). Informed consent was waived in this study as it was a retrospective review of de-identified data only.
Results
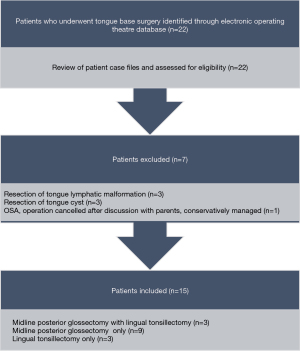
Twenty-two consecutive patients underwent tongue base surgery from 1st January 2007 to 30th June 2021 and seven were excluded based on the eligibility criteria (Figure 1). A total of 15 patients (73.3% male; median age, 8 years; age range, 10 months–15 years) were included in this study.
Most patients (80%) had moderate to severe OSA prior to TBR. Eleven patients had a syndromic diagnosis, seven had a history of cardiopulmonary disease and two had a history of endocrine disorder (Table 2). The median preoperative BMI of the group was 20.2 [standard deviation (SD), 8.17; range, 15.5–45.1] kg/m2. According to the Centers for Disease Control and Prevention (CDC) Clinical Growth Charts (29), 13.3% of patients were overweight (BMI 85th to <95th percentile) and 33.3% were obese (BMI ≥95th percentile). The most common clinical presentations were snoring (93.3%), restlessness (93.3%) and daytime fatigue or inattention (60.0%) (Table 3).
Table 2
| Patient characteristics | Data |
|---|---|
| Number of patients | 15 |
| Gender (M/F), n (%) | 11 (73.3)/4 (26.7) |
| Age† (years), median (SD) [range] | 8 (4.66) [0.83–15] |
| BMI‡ (kg/m2), median (SD) [range] | 20.2 (8.17) [15.5–45.1] |
| BMI percentile, median (SD) [range] | 71 (35.2) [3–99] |
| BMI risk, n (%) | |
| Underweight (BMI <5th percentile) | 1 (6.67) |
| Healthy weight (BMI 5th to <85th percentile) | 7 (46.7) |
| Overweight (BMI 85th to <95th percentile) | 2 (13.3) |
| Obese (BMI ≥95th percentile) | 5 (33.3) |
| ASA, n (%) | |
| I | 0 |
| II | 4 (26.7) |
| III | 11(73.3) |
| IV | 0 |
| Syndromic diagnosis, n (%) | 11 (73.3) |
| DS | 6 |
| Rubenstein-Taybi syndrome | 2 |
| Beckwith-Wiedemann syndrome | 1 |
| Chromosome 6q duplication | 1 |
| Prader-Willi syndrome | 1 |
| Cardiac/pulmonary disorders, n (%) | 7 (46.7) |
| AVSD/VSD/ASD | 5 |
| Repaired tetralogy of Fallot | 1 |
| Bicuspid AV | 1 |
| Endocrine/metabolic disorders, n (%) | 2 (13.3) |
| T2DM | 1 |
| Primary growth hormone deficiency on hormone replacement | 1 |
| Craniofacial abnormalities, n (%) | 2 (13.3) |
| Retrognathia | 2 |
†, age at time of operation; ‡, BMI as calculated by CDC’s BMI Percentile Calculator for Child and Teen ≥2 years of age; BMI was not applicable in children under 2 years old, whose weight plotted against age on an age-appropriate growth chart. M, male; F, female; SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists’ Physical Status Classification System; DS, down syndrome; AVSD, atrioventricular septal defect; VSD, ventricular septal defect; ASD, atrial septal defect; AV, atrioventricular; T2DM, type 2 diabetes mellitus; CDC, Centers for Disease Control and Prevention.
Table 3
| Presenting history | Frequency |
|---|---|
| Common clinical presentation, n (%) | |
| Snoring | 14 (93.3) |
| Restlessness | 14 (93.3) |
| Daytime fatigue or inattention | 9 (60.0) |
| Pauses | 8 (53.3) |
| Mouth breathing | 6 (40.0) |
| Gasping | 3 (20.2) |
| Prior AT, n (%) | 12 (80.0) |
| Prior trial of PAP, n (%) | 10 (66.7) |
| Preoperative PAP tolerant, n (%) | 5 (33.3) |
AT, adenotonsillectomy; PAP, positive airway pressure.
Pre- and postoperative PSG were performed in 12 patients. Two patients did not have PSG: one had preoperative inpatient overnight oximetry with significant desaturations, suggestive of severe OSA; the other had obstructive symptoms consistent with severe OSA, requiring urgent surgery; both had dramatic clinical resolution of symptoms postoperatively and a postoperative PSG was not required. One patient had mild OSA on preoperative PSG and did not require a postoperative PSG due to clinical resolution of symptoms. Individual patient demographics, comorbidities, surgical procedures, and pre- and postoperative PSG results are summarized in Table 4. Pre- and postoperative OSA scores are summarized in Table 5.
Table 4
| No. | Age† (years) | Gender | Preoperative BMI‡ percentile (BMI risk) | Co-morbidities (ASA grade) | Procedures | Preoperative OAHI (LSAT) | Postoperative OAHI (LSAT) | Change in OAHI | Percentage reduction in preoperative OAHI (%) | Successful outcome§, comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | F | 93 (overweight) | Nil (ASA II) | MPG, LT, RA, CIT | N/A (inpatient overnight oximetry suggestive of severe OSA) | N/A (Significant clinical improvement, no residual symptoms, no PAP requirement) | N/A | N/A | Yes |
| 2 | 12 | F | 99 (obese) | DS, T2DM, intellectual disability, GDD, recurrent laryngeal papilloma, congenital sensorineural hearing loss (ASA III) | MPG, LT | 30.9 (68%) | 55.2 (52%) (postoperative weight gain of 15 kg) | −24.3 | −78.6 | No, referred for PAP therapy |
| 3 | 13 | M | 97 (obese) | DS, AVSD repair, recurrent bronchitis (ASA II) | MPG, LT, turbinoplasty | 7.0 (87%) | 24.9 (80%) | −17.9 | −256 | No, referred for PAP therapy, improved PAP tolerance |
| 4 | 10 months | M | 5 (healthy) | DS, PDA and VSD repair, pulmonary hypertension, choanal atresia repair, Hirschsprung disease, duodenal atresia, PEG fed, hepatitis C, GDD (ASA III) | MPG, AT | 10.4 (64%) | 0.2 (93%) | 10.2 | 98.1 | Yes |
| 5 | 5 | M | 93 (overweight) | Rubenstein-Taybi, congenital single kidney, undescended testes orchidopexy, ureteric reimplantation, GDD (ASA III) | MPG, AT, CIT | 33.5 (76%) | 1 (86%) | 32.5 | 97.0 | Yes |
| 6 | 2 | M | 18 (healthy) | Beckwith-Wiedemann, bicuspid AV valve, anterior cerebral artery infraction with left encephlomalacia and frontal focal epilepsy, GMFCS III spastic diplegic cerebral palsy, tracheomalacia, right duplex kidney with vesicoureteric reflux, GDD (ASA III) | MPG | 20.9 (73%) | 0.5 (98%) | 20.4 | 97.6 | Yes |
| 7 | 7 | F | 98 (obese) | DS, small unrepaired muscular ASD, laryngomalacia, GDD (ASA II) | MPG | 39.8 (82%) | 26.5 (84%) | 13.3 | 33.4 | No, referred for PAP therapy, improved PAP tolerance |
| 8 | 8 | M | 54 (healthy) | Prader-Willi, primary growth hormone deficiency on Genotropin, hypotonia, GDD, autism spectrum disorder (ASA III) | MPG, RA | 10.1 (85%) | 0.3 (72%) | 9.8 | 97.0 | Yes |
| 9 | 9 | M | 40 (healthy) | Nil (ASA III) | LT, RA | N/A (symptoms consistent with severe OSA, urgent elective surgery) | N/A (significant clinical improvement, mild residual symptoms, no PAP requirement) | N/A | N/A | Yes |
| 10 | 8 | M | 95 (obese) | Rubenstin-Taybi, small unrepaired VSD, chronic cough, bilateral undescended testes, GDD (ASA III) | LT | 20.9 (79%) | 6.1 (86%) | 14.8 | 70.8 | Yes |
| 11 | 15 | M | 94 (overweight) | Refractory epilepsy with corpus callostomy, intellectual disability, retrognathia, narrow maxilla (ASA III) | MPG + turbinoplasty | 6.7 (88%) (underestimated hypopnoeic and apnoeic events due to epileptiform activity) | 62.8 (79%) | N/A | N/A | No, some clinical improvement, subsequent SARME |
| 12 | 15 | F | 71 (healthy) | DS, tetralogy of Fallot repair (ASA III) | MPG | 13.5 (66%) (underestimated due to many sub-criterion events) | 0 (81%) | 13.5 | 100 | Yes |
| 13 | 1.5 | M | 3 (underweight) | Chromosome 6q duplication, ex premature twin 32/40, laryngotracheobronchomalacia, tracheostomy and ventilator dependent, retrognathia and macroglossia, deep laryngeal cleft, immune deficiency, recurrent LRTI, PEJ fed, right ureteropelvic junction malrotation and obstruction, bilateral hernia repair, GDD (ASA III) | MPG | 3.6 (81%) (sleep study performed with NPA due to severity of OSA) | 4.1 (81%) (sleep study performed with tracheostomy capped) | N/A | N/A | Decannulated |
| 14 | 8 | M | 57 (healthy) | DS, incomplete AVSD and membranous subaortic stenosis repair, immune deficiency, recurrent croup, tracheomalacia, severe anxiety (ASA III) | LT + RA | 2.1 (82%) | N/A (significant clinical improvement, mild residual symptoms, no PAP requirement, will not tolerate repeat PSG due to severe anxiety) | N/A | N/A | Yes |
| 15 | 5 | M | 60 (healthy) | FASD, ex premature 35/40, central hypotonia, recurrent aspiration pneumonia, PEG fed, laryngomalacia (ASA II) | MPG | 57.6 (45%) | 0.3 (97%) | 57.3 | 99.5% | Yes |
†, age at time of operation; ‡, BMI as calculated by CDC’s BMI Percentile Calculator for Child and Teen ≥2 years of age; BMI was not applicable in the 10 months old patient, whose weight plotted against age on an age-appropriate male growth chart was in the 5th percentile; §, successful outcome was postoperative resolution of rOSA, defined by postoperative OAHI <5 or ≥50% reduction in preoperative OAHI or complete cessation of PAP therapy. F, female; M, male; BMI, body mass index; ASA, American Society of Anesthesiologists’ Physical Status Classification System; DS, down syndrome; T2DM, type 2 diabetes mellitus; GDD, global developmental delay; AVSD, atrioventricular septal defect; PDA, patent ductus arteriosus; VSD, ventricular septal defect; PEG, percutaneous endoscopic gastrostomy; AV, atrioventricular; GMFCS, Gross Motor Function Classification System; ASD, atrial septal defect; LRTI, lower respiratory tract infection; PEJ, percutaneous endoscopic jejunostomy; FASD, fetal alcohol spectrum disorders; MPG, midline posterior glossectomy; LT, lingual tonsillectomy; RA, revision adenoidectomy; CIT, cautery of inferior turbinates; AT, adenotonsillectomy; OAHI, obstructive apnoea hypopnoea index; LSAT, low oxyhaemoglobin desaturation; N/A, not applicable; OSA, obstructive sleep apnoea; NPA, nasopharyngeal airway; PAP, positive airway pressure; PSG, polysomnography; SARME, surgically assisted rapid maxillary expansion; CDC, Centers for Disease Control and Prevention; rOSA, refractory obstructive sleep apnoea.
Table 5
| OSA severity score | Preoperative (n=15), n (%) | Postoperative (n=15), n (%) |
|---|---|---|
| Normal | 0 | 9 (60.0) |
| Mild | 2 (13.3) | 1 (6.67) |
| Moderate | 2 (13.3) | 1 (6.67) |
| Severe | 11(73.3) | 4 (26.7) |
OSA, obstructive sleep apnoea.
Ten patients (66.7%) had complete resolution of rOSA (postoperative OAHI <5, ≥50% reduction in preoperative OAHI or complete cessation of PAP therapy). Three patients had persistent OSA and were referred for further PAP therapy, of which, two had improved PAP tolerance and one (patient 2) had worse OSA due to 15 kg weight gain (Figure 2). One patient (patient 11) was referred for surgically assisted rapid maxillary expansion for correction of retrognathia and narrow maxilla, and one (patient 13) was decannulated after a repeat PSG with capped tracheostomy tube that demonstrated mild OSA.
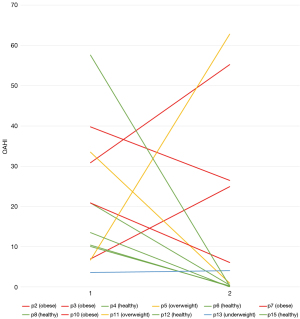
Statistical analysis was performed to compare the pre- and postoperative PSG results in 12 (Table 6). The preoperative mean OAHI was 21.2 (SD, 16.3; range, 3.6–57.6) and the postoperative mean OAHI was 15.2 (SD, 22.6; range, 0–62.8). The mean percentage reduction in OAHI was 83.9 and the decrease in postoperative OAHI was not statistically significant (P=0.480).
Table 6
| Patient variables | Preoperative | Postoperative | t value | P value |
|---|---|---|---|---|
| OAHI (n=12), median | 17.2 | 2.55 | N/A | N/A |
| OAHI (n=12), mean (SD) [range] | 21.2 (16.3) [3.6–57.6] | 15.2 (22.6) [0–62.8] | 2.20 | 0.480 |
| Change in OAHI (n=12), mean (SD) [range] | – | 11.75 (28.8) [−56.1 to 57.3] | N/A | N/A |
| Percentage reduction in OAHI (n=12), mean (SD) [range] | – | 83.9 (272.4) [−837.3 to 100.0] | N/A | N/A |
| Subgroup analyses | ||||
| Obese OAHI (n=4), mean (SD) [range] | 25.9 (17.0) [7–39.8] | 35.5 (17.1) [6.1–55.2] | 3.18 | 0.753 |
| Overweight OAHI (n=2), mean (SD) [range] | 20.1 (17.9) [6.7–33.5] | 31.9 (36.0) [1–62.8] | 12.7 | 0.834 |
| Healthy OAHI (n=5), mean (SD) [range] | 35.6 (31.2) [13.5–57.6] | 0.15 (0.212) [0–0.3] | 2.78 | 0.0682 |
| Underweight OAHI (n=1), median | 3.6 | 4.1 | N/A | N/A |
| Male OAHI (n=9), mean (SD) [range] | 10.4 (9.22) [3.6 – 57.6] | 24.33(33.3) [0.3-62.8] | 2.31 | 0.481 |
| Female OAHI (n=3), mean (SD) [range] | 35.33 (20.9) [13.5 – 39.8] | 40.85 (27.6) [0-55.2] | 4.30 | 0.953 |
| Syndromic OAHI (n=10), mean (SD) [range] | 8.55 (7.00) [3.6–13.5] | 2.05 (2.90) [0–4.1] | 2.26 | 0.218 |
| Non syndromic OAHI (n=2), mean (SD) [range] | 6.7 (4.74) [6.7–57.6] | 62.8 (44.4) [0.3–62.8] | 12.7 | 0.993 |
OAHI, obstructive apnoea hypopnoea index; SD, standard deviation; N/A, not applicable.
Subgroup analysis was performed to compare the PSG results based on BMI, gender, and the presence of a syndromic diagnosis. Patients were divided into four groups based on their BMI risk category: underweight (BMI <5th percentile, n=1), healthy weight (BMI 5th to <85th percentile, n=4), overweight (BMI 85th to <95th percentile, n=2), and obese (BMI ≥95th percentile, n=4). Patients in the healthy group had the greatest reduction in mean OAHI post-operatively (P=0.0682). Neither gender nor the presence of a syndromic diagnosis appeared to be significant factors.
Successful outcome was achieved in 66.7% (10/15) of patients in this study: 33.3% (1/3) patients who underwent MPG with LT, 66.7% (6/9) patients who underwent MPG, and all (3/3) patients who underwent LT.
There was no mortality among the patients in this study. Patient 9 had a prolonged ICU admission due to traumatic intubation (grade IV view on laryngoscopy) and intraoperative desaturations and had a planned extubation in theatre 3 days after surgery. No significant complications such as injury to lingual or hypoglossal nerves, significant vascular injury, postoperative bleeding requiring re-intervention, or obstructive airway symptoms requiring tracheostomy occurred in this study.
Discussion
Within the limitations of a retrospective study with a small patient cohort, this study found that children with healthy weight (BMI 5th to <85th percentile) demonstrated consistent improvement in rOSA after TBR, whereas children with abnormally increased or decreased BMI had variable outcomes. This appears to be independent of patients’ gender and syndromic diagnoses.
BMI and TBR in rOSA
The finding of healthy BMI as a positive predictor of outcome in rOSA after TBR surgery is consistent with current evidence (16,18,24,26,30). Obesity is independently correlated to lingual tonsil hypertrophy in children, thereby contributing to rOSA (31-33). Additionally, the oropharyngeal space is compressed by adipose tissue, resulting in a decrease in its cross-sectional area and an exponential increase in airway resistance. In the present study, we divided patients into four BMI risk categories according to the CDC Clinical Growth Charts (29). We found that obese and overweight children had less favorable outcomes with MPG and/or LT, which is consistent with the findings of Propst et al. (20). The worsening of rOSA symptoms in patient 2 in the setting of significant postoperative weight gain supports this finding.
We recommend that BMI be taken into consideration in the preoperative workup and counselling of children with rOSA and aggressive weight loss programs in a multidisciplinary setting be encouraged prior to surgery.
Safety and efficacy of LT and MPG
LT has been found to be an effective surgical option for children with rOSA due to lingual tonsil hypertrophy (25). A recent meta-analysis of LT in the treatment of paediatric OSA found that it resulted in significant improvements in minimum oxygen saturation by 6% [95% confidence interval (CI): 2.7–9.2%] and mean AHI by 8.9/hr (95% CI: −12.6/hr to −5.2/hr) (25). While data on the complications of LT are limited, the rate of serious complications appear to be low. The same study found an overall complication rate of 6.84%, including three patients with airway obstruction, postoperative bleeding, and pneumonia (25).
All studies on MPG have included other airway procedures in paediatric rOSA patients (20-24). This makes the interpretation of complications such as postoperative bleeding and airway obstruction difficult. However, MPG appears to be a safe option for treating paediatric patients with rOSA due to tongue base obstruction (20-24).
We found TBR involving MPG and LT to be safe and effective with a success rate of 66.7% for all procedures combined and no significant complication was observed in this series. While all patients with rOSA should be considered for a trial of PAP, patients with definite tongue base obstruction as demonstrated on DISE or Cine MRI may benefit from targeted surgical therapy. Even among patients with partial improvement, the reduction in tongue base resistance has been shown to improve tolerance of PAP therapy (20). This was observed in patients 3 and 7.
As demonstrated in Table 4, many of our patients have a complex list of comorbidities as well as social and psychological issues that impede their tolerance and/or compliance with PAP therapy. The clinical decision tree in these patients is complex and require extensive multidisciplinary input and discussion. At our institution, children with rOSA are referred to a Complex Airway Team service that includes otolaryngologists, sleep physicians, paediatricians, craniofacial surgeons, speech pathologists, dietitians, social workers, and advanced scope nurse practitioners. Syndromic children with rOSA particularly benefit from this model of care, in which they are referred early for multidisciplinary discussion, coordination of care and intervention.
Limitations
This study has several limitations. First, the study was retrospective and not all patients had pre- and postoperative PSG. Second, the inclusion of other concurrent procedures such as revision adenoidectomy, cautery of inferior turbinates and turbinoplasty may contribute to the degree of improvement seen in our cohort. Finally, the study was limited by the small sample size. However, MPG and LT are uncommon procedures and there are only 52 cases of MPG reported in the literature. Large, multi-institutional studies are required to confirm the findings of this study.
Conclusions
TBR using MPG and/or LT appears to be a safe and effective treatment option in paediatric rOSA. A healthy weight (BMI 5th to <85th percentile) was associated with a positive outcome and larger studies are required to confirm this finding. A multidisciplinary approach to weight loss and preoperative medical optimization is recommended to maximise the benefits of surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-21-35/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-21-35/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-21-35/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-21-35/coif). SV received honorarium from Smith-Nephew for educational events. SV serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflict of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the local research ethics committee (approval number: 26695). Informed consent was waived in this study as it was a retrospective review of de-identified data only.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Australasian Sleep Association. Obstructive Sleep Apnoea in Childhood. 2019. Available online: https://sleep.org.au/common/Uploaded%20files/Public%20Files/Professional%20resources/Paed%20resources/Obstructive%20Sleep%20Apnoea%20in%20Children.pdf (Accessed on May 20, 2020).
- Chan J, Edman JC, Koltai PJ. Obstructive sleep apnoea in children. Am Fam Physician 2004;69:1147-54. [PubMed]
- Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366-76. [Crossref] [PubMed]
- Lee CH, Hsu WC, Chang WH, et al. Polysomnographic findings after adenotonsillectomy for obstructive sleep apnoea in obese and non-obese children: a systematic review and meta-analysis. Clin Otolaryngol 2016;41:498-510. [Crossref] [PubMed]
- O'Brien LM, Sitha S, Baur LA, et al. Obesity increases the risk for persisting obstructive sleep apnea after treatment in children. Int J Pediatr Otorhinolaryngol 2006;70:1555-60. [Crossref] [PubMed]
- Hoeve LJ, Pijpers M, Joosten KF. OSAS in craniofacial syndromes: an unsolved problem. Int J Pediatr Otorhinolaryngol 2003;67:S111-3. [Crossref] [PubMed]
- Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010;182:676-83. [Crossref] [PubMed]
- ElMallah M, Bailey E, Trivedi M, et al. Pediatric Obstructive Sleep Apnea in High-Risk Populations: Clinical Implications. Pediatr Ann 2017;46:e336-9. Erratum in: Pediatr Ann 2017;46:e436. [PubMed]
- Mitchell RB. Adenotonsilecotmy for Obstructivd Sleep Apnoea in Children: Outcome Evaluated by Pre- and postoperative polysomnography. Laryngoscope 2007;117:1844-54. [Crossref] [PubMed]
- Donnelly LF, Shott SR, LaRose CR, et al. Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRI. AJR Am J Roentgenol 2004;183:175-81. [Crossref] [PubMed]
- Bertapelli F, Pitetti K, Agiovlasitis S, et al. Overweight and obesity in children and adolescents with Down syndrome-prevalence, determinants, consequences, and interventions: A literature review. Res Dev Disabil 2016;57:181-92. [Crossref] [PubMed]
- Truong MT, Woo VG, Koltai PJ. Sleep endoscopy as a diagnostic tool in pediatric obstructive sleep apnea. Int J Pediatr Otorhinolaryngol 2012;76:722-7. [Crossref] [PubMed]
- Xanthopoulos MS, Kim JY, Blechner M, et al. Self-Efficacy and Short-Term Adherence to Continuous Positive Airway Pressure Treatment in Children. Sleep 2017; [Crossref] [PubMed]
- Parmar A, Baker A, Narang I. Positive airway pressure in pediatric obstructive sleep apnea. Paediatr Respir Rev 2019;31:43-51. [Crossref] [PubMed]
- Lin AC, Koltai PJ. Persistent pediatric obstructive sleep apnea and lingual tonsillectomy. Otolaryngol Head Neck Surg 2009;141:81-5. [Crossref] [PubMed]
- Abdel-Aziz M, Ibrahim N, Ahmed A, et al. Lingual tonsils hypertrophy; a cause of obstructive sleep apnea in children after adenotonsillectomy: operative problems and management. Int J Pediatr Otorhinolaryngol 2011;75:1127-31. [Crossref] [PubMed]
- DeMarcantonio MA, Senser E, Meinzen-Derr J, et al. The safety and efficacy of pediatric lingual tonsillectomy. Int J Pediatr Otorhinolaryngol 2016;91:6-10. [Crossref] [PubMed]
- Skirko JR, Jensen EL, Friedman NR. Lingual tonsillectomy in children with Down syndrome: Is it safe? Int J Pediatr Otorhinolaryngol 2018;105:52-5. [Crossref] [PubMed]
- Prosser JD, Shott SR, Rodriguez O, et al. Polysomnographic outcomes following lingual tonsillectomy for persistent obstructive sleep apnea in down syndrome. Laryngoscope 2017;127:520-4. [Crossref] [PubMed]
- Propst EJ, Amin R, Talwar N, et al. Midline posterior glossectomy and lingual tonsillectomy in obese and nonobese children with down syndrome: Biomarkers for success. Laryngoscope 2017;127:757-63. [Crossref] [PubMed]
- Wootten CT, Chinnadurai S, Goudy SL. Beyond adenotonsillectomy: outcomes of sleep endoscopy-directed treatments in pediatric obstructive sleep apnea. Int J Pediatr Otorhinolaryngol 2014;78:1158-62. [Crossref] [PubMed]
- Ulualp S. Outcomes of Tongue Base Reduction and Lingual Tonsillectomy for Residual Pediatric Obstructive Sleep Apnea after Adenotonsillectomy. Int Arch Otorhinolaryngol 2019;23:e415-21. [Crossref] [PubMed]
- Thottam PJ, Govil N, Duvvuri U, et al. Transoral robotic surgery for sleep apnea in children: Is it effective? Int J Pediatr Otorhinolaryngol 2015;79:2234-7. [Crossref] [PubMed]
- Maturo SC, Mair EA. Submucosal minimally invasive lingual excision: an effective, novel surgery for pediatric tongue base reduction. Ann Otol Rhinol Laryngol 2006;115:624-30. [Crossref] [PubMed]
- Kang KT, Koltai PJ, Lee CH, et al. Lingual Tonsillectomy for Treatment of Pediatric Obstructive Sleep Apnea: A Meta-analysis. JAMA Otolaryngol Head Neck Surg 2017;143:561-8. [Crossref] [PubMed]
- Camacho M, Noller MW, Zaghi S, et al. Tongue surgeries for pediatric obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2017;274:2981-90. [Crossref] [PubMed]
- Australasian Sleep Association. ASTA/ASA Addendum to AASM Guidelines for recording and scoring of paediatric sleep. 2011.
- Berry R, Brroks R, Gamaldo C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (version 2.2). Darien: American Academy of Sleep Medicine, 2015.
- Centers for Disease Control and Prevention. What is BMI? 2018. Available online: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html (Accessed on May 20, 2020).
- Scheffler P, Wolter NE, Narang I, et al. Surgery for Obstructive Sleep Apnea in Obese Children: Literature Review and Meta-analysis. Otolaryngol Head Neck Surg 2019;160:985-92. [Crossref] [PubMed]
- Sung MW, Lee WH, Wee JH, et al. Factors associated with hypertrophy of the lingual tonsils in adults with sleep-disordered breathing. JAMA Otolaryngol Head Neck Surg 2013;139:598-603. [Crossref] [PubMed]
- Hwang MS, Salapatas AM, Yalamanchali S, et al. Factors associated with hypertrophy of the lingual tonsils. Otolaryngol Head Neck Surg 2015;152:851-5. [Crossref] [PubMed]
- Guimaraes CV, Kalra M, Donnelly LF, et al. The frequency of lingual tonsil enlargement in obese children. AJR Am J Roentgenol 2008;190:973-5. [Crossref] [PubMed]
Cite this article as: Zhen E, Locatelli Smith A, Herbert H, Vijayasekaran S. Midline posterior glossectomy and lingual tonsillectomy in children with refractory obstructive sleep apnoea: factors that influence outcomes. Aust J Otolaryngol 2022;5:24.