
Xylitol nasal preparations in sinonasal disease: a literature review and meta-analysis
Introduction
Xylitol is a naturally occurring chemical compound which exists as a five-carbon sugar. It has been used as a sugar substitute for products such as chewing gum, toothpaste and confectionary (1). Its metabolism is free from the influence of insulin therefore has negligible effects of blood sugar.
Xylitol has been shown to exhibit antimicrobial properties with several studies demonstrating that this is owed to its inherent ability to enhance the innate immune system by altering the salt concentration of the airway surface liquid thereby increasing the effect of endogenous antimicrobials such as lysozymes, lactoferrin and beta defensins (2,3). It also acts as an anti-biofilm agent through inhibition of key enzymes (4-6).
From a rhinologic perspective, xylitol has shown promise in the treatment of sinonasal disease with animal studies demonstrating its efficacy in reducing chronic rhinosinusitis (CRS) biofilms (7) and enhancing bacterial killing in nasal and sinus mucosa (8). Specifically, it has effects on several pathogens commonly implicated in sinonasal disease, directly affecting the growth of Streptococcus pneumoniae and Haemophilus influenzae whilst being able to dissolve the biofilm structure of Pseudomonas aeruginosa. As a result, xylitol has now been found as an additive to saline solution in commercially available nasal sprays and sinus rinses (9).
CRS is a prevalent condition in Australia, it carries a high burden on our healthcare system accounting for 1.4% of all general practice encounters. It has been estimated that the yearly cost of CRS in terms of lost productivity is approximately $10,000 AUD per patient per annum (10). Despite significant advancements in medical and surgical treatments a high burden of treatment refractory symptoms and recurrence remain (11).
The goal of this review is to assess evidence pertaining to xylitol nasal preparations and its efficacy in the treatment of sinonasal disease in both pre and post-operative patient groups. We present the following article in accordance with the PRISMA reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-21-45/rc) (12).
Methods
A systematic review of the literature relating to the use of xylitol in treating sinonasal disease was performed.
Searching
We performed an electronic search of the literature via Medline, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) from their respective dates of inception up until September 2021. The electronic search was performed using a combination of keywords consisting of ‘xylitol’, ‘nasal’, ‘sinus’, ‘sinusitis’ and ‘rhinitis’. Two reviewers independently screened the search results and the bibliographies of each article were also hand searched for any further relevant trials. Duplicate results were removed. No automation tools were used in this process.
Population inclusion criteria comprised of adult patients with sinonasal disease (rhinosinusitis with or without nasal polyposis, rhinitis or inferior turbinate hypertrophy). The intervention consisted of xylitol additives to nasal topical medications in comparison to any other nasal preparations including saline or conservative treatment. Only randomised controlled trials were included in this review.
Outcome measure
The primary outcome measure was the difference in 22-item Sinonasal Outcome Test (SNOT-22) scores between the intervention (xylitol) versus the comparison (saline) group before and after use. The SNOT-22 is a validated questionnaire designed to assess the burden of CRS symptomology. The questionnaire consists of 22 questions each scored on a Likert scale ranging from 0 (no problem) to 5 (Problem as bad as it can be). The higher the SNOT-22 score the higher the burden of disease and vice versa. The mean SNOT-22 scores between pre-and post-treatment from the Xylitol group would then be subtracted from equivalent score from the Saline group giving a quantifiable measure of Xylitol’s effects. A more negative value would suggest xylitol having a greater effect whilst a more positive value would suggest that saline has a greater effect.
Our results would then be compared against the Minimal Clinically Important Difference (MCID) value for the SNOT-22 score. The MCID score is defined as the minimal required change in a score on an outcome instrument (in our case SNOT-22) that corresponds to a patient’s perception of beneficial change (13). The MCID value for SNOT-22 was defined a priori as 8.9 (14) with later similar studies corroborating this value (15,16).
Selection and data collection process
Non-English texts were excluded as well as studies which did not utilise validated outcome measures such as the SNOT-22. A risk of bias assessment was performed across all included studies by using the revised Cochrane risk-of-bias tool for randomised trials. Full text articles for each of the relevant studies were obtained for further review and data collection. Data was compiled on study design (duration, inclusion and exclusion criteria, population size, intervention type and control), population characteristics and outcome measures (mean difference in SNOT-22 score).
Statistical analysis
In instances where the standard deviation or 95% confidence intervals were not provided in the text, they were calculated from the provided data using the relevant statistical formulae. Data meta-analysis was performed using Review Manager 5.4. The SNOT-22 score mean difference was pooled using a random effect model. Statistical heterogeneity between the studies was evaluated using the I2 statistic. A subgroup analysis was performed with the outcome measure compared between patients who have undergone surgery versus those who have not. We further divided the post-surgical subgroup into patients who have had endoscopic sinus surgery (ESS) compared to other operative procedures such as septoplasty. Analysis was planned to be performed using a random effects model with a 95% confidence interval and P<0.05 pre-specified as being statistically significant.
Results
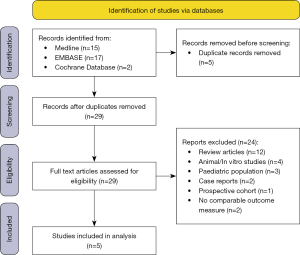
A total of 34 articles were identified after our electronic search, 5 of the articles were excluded due to being duplicates. Given the small number of articles yielded from our search the full text of 29 articles were then reviewed, of which 22 were excluded. Reasons for exclusion included, non-randomised control trial, animal/in vitro studies and a paediatric study population. A total of 7 eligible RCTs were identified however 2 had to be excluded from further analysis as unlike the other RCTs, they did not use the validated and comparable SNOT-22 score as their outcome measure to assess intervention effect.
Cingi et al. used a visual analog scale (VAS) where they asked participants to mark their overall ‘sinonasal wellbeing’ as well as assessing quality of life by means of using the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ). It should be noted that they also employed rhinomanometry as an objective measure of treatment effect (17), however this was the only study which employed this method and therefore the results would not have been comparable across the other groups. Sanchez-Gonzalez et al. used the VAS score as well as the daily symptoms score (DSS) as their outcome measure (18).
Therefore, a total of 5 articles were identified for inclusion into our analysis. The PRISMA flow diagram for identification of studies for inclusion is shown in Figure 1 (12).
The characteristics of the included studies have been summarised and can be found in Table 1. Three out of the 5 included studies were blinded. Hayer et al. did not attempt to blind their participants as they reported that participants could taste the difference in irrigation solutions, the outcome assessors, data entry personnel and statistician were however blind to the allocation group (19). The xylitol solutions used were either 1.6% or 5% wt/vol, the mode of delivery remained consistent across all studies. There was considerable variability in frequency of treatment ranging from once daily to three times daily irrigations and similarly regarding duration of treatment with the shortest trial lasting 26 days and the longest trial lasting 26 weeks.
Table 1
| Authors [year] | Study design | Population [n] | Solutions used | Method of delivery | Duration of treatment | Outcome measures |
|---|---|---|---|---|---|---|
| Weissman et al. [2011] | Randomised double blinded crossover trial (xylitol vs. saline) | CRS patients [20] | Xylitol (5% wt/vol); Saline (0.9% wt/vol) | Irrigation | 3 days washout prior to commencement of treatment. Once daily irrigations for 10 days followed by 3 days washout and once daily irrigation with alternate irrigant | SNOT-22 score; VAS score |
| Lin et al. [2017] | Randomised double blinded trial (xylitol vs. saline) | CRS patients with previous ESS [30]; 15 per arm | Xylitol (5% wt/vol); Saline (0.9% wt/vol) | Irrigation | Once daily irrigations for 30 days | SNOT-22 score; VAS score; Nasal NO; iNOS mRNA |
| Kim et al. [2019] | Randomised double blinded crossover trial (xylitol vs. saline) | Patients with sinonasal disease who underwent septoplasty/ESS/both [100]; 50 per arm | Xylitol (1.6% wt/vol); Saline (0.9% wt/vol) | Irrigation | Three times daily irrigations for 14 days followed by 7 days washout and three times daily irrigations for 14 days with alternate irrigant | NOSE score; SNOT-22 score; VAS score; Modified Lund-Kennedy score |
| Rabago et al. [2020] | Randomised control trial (xylitol vs. saline vs. control) | Patients meeting criteria for Gulf War Illness with moderate to severe chronic rhinosinusitis [40] | Xylitol (1.6% wt/vol); Saline (2% wt/vol) | Irrigation | Twice daily irrigations for 26 weeks | SNOT-22 score; MFI score |
| Sylvia et al. [2020] | Prospective randomised controlled study (xylitol vs. saline) | Patients with CRSwNP or CRSsNP refractory to medical treatment [52] | Xylitol (1.6% wt/vol); Saline (0.9% wt//vol) | Irrigation | Three times daily irrigations for 30 days | VAS score; SNOT-22 score; NOSE score |
CRS, chronic rhinosinusitis; ESS, endoscopic sinus surgery; SNOT-22, 22-item Sinonasal Outcome Test; VAS, visual analog scale; NO, nitric oxide; iNOS, inducible nitric oxide synthase; NOSE, nasal obstruction symptom evaluation; MFI, multidimensional fatigue inventory; CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps.
A risk of bias assessment was performed across all the included studies with use of the modified Cochrane Collaboration tool. Bias outcome was assessed as a judgement (either high, low or unclear) across multiple domains through which bias may be introduced into the trial (20). The results of which can be found in Table 2.
Table 2
| Authors [year] | Random sequence generation | Allocation concealment | Selective reporting | Other sources of bias | Blinding (participants and personnel) | Blinding (outcome assessment) | Incomplete outcome data |
|---|---|---|---|---|---|---|---|
| Weissman et al. [2011] | Low | Low | Unclear | High | Low | Low | Low |
| Lin et al. [2017] | Low | Low | Unclear | Low | Low | Unclear | Low |
| Kim et al. [2019] | Low | Low | Unclear | Low | Low | Unclear | Low |
| Rabago et al. [2020] | Low | High | Unclear | Low | High | High | Low |
| Sylvia et al. [2020] | Low | Low | Unclear | Low | Low | Low | Low |
All the included studies adequately described their method of random sequence allocation to produce comparable intervention and control groups, and this was demonstrated in the comparable demographic characteristics as shown in each of the articles.
Selective reporting bias was a domain that was assessed as being unclear across all the trials as there was insufficient data to permit judgement. We did not find any specific mention in any of the study protocols which addressed whether the trial would be analysed in concordance with the finalized pre specified plan before the outcome data was available for analysis. It should be noted however that according to the risk of bias assessment tool that we used, most studies were expected to fall into this category.
There was a total of 5 RCTs that included the mean change in SNOT-22 score between xylitol and saline as their outcome measure, thereby permitting a meta-analysis to be performed (Figure 2). The overall pooled mean difference in SNOT-22 scores between xylitol versus saline was −7.77 (95% CI: −10.89 to −4.65, P<0.00001). A random effects model was used to pool mean difference given significant heterogeneity seen across all included studies (I2=85%).

Furthermore, a subgroup analysis was performed between post-operative patients versus non operative patients. The post- operative patients were divided into an ESS group and a non ESS group. Kim et al. was the only trial that included a post-surgical patient population that did not have ESS, they underwent a septoplasty (21).
In the post-operative ESS group, the pooled mean difference in SNOT-22 scores between xylitol versus saline was −11.23 (95% CI: −12.97 to −9.48, P<0.00001). A random effects model was used to pool mean difference given moderate heterogeneity in the included studies (I2=37%). In the post-operative non ESS group, the mean difference in SNOT-22 score between xylitol versus saline was 0.40 (95% CI: –7.47 to 8.27) this result was not statistically significant. In the non-surgical group the pooled mean difference in SNOT-22 scores between xylitol versus saline was −4.99 (95% CI: −8.96 to −1.02, P=0.01). A random effects model was used to pool mean difference given significant heterogeneity in the included studies (I2=51%).
Compared to the predefined MCID score of 8.9, the post-surgical ESS subgroup met this threshold with a score of 11.23, whereas the non-surgical subgroup failed to meet the threshold with a score of 4.99. The post-surgical non ESS subgroup fell well short of the MCID score with a positive mean difference score of 0.40, suggesting that xylitol does not make a significant difference in this subgroup.
Discussion
Xylitol has several advantages, it is cost effective, readily available over the counter and has been proven to be safe for human use (22). It has a small side effect profile and was reported across all studies to be well tolerated by the participants; no participants were removed from trials as a result of xylitol side effects.
This review identified several RCTs which assessed the effectiveness of xylitol nasal preparations in the treatment of sinonasal disease. The meta-analysis and consequent subgroup analysis found evidence that xylitol may be more effective than normal saline at reducing the burden of disease and symptomology in CRS. In comparison to normal saline, xylitol was associated with a greater reduction in SNOT-22 scores (−7.77, 95% CI: −10.89 to −4.65, P<0.00001). In the post-surgical ESS subgroup, the average reduction was greater (−11.23, 95% CI: −12.97 to −9.48, P<0.00001) whilst in the non-surgical subgroup the difference was less noticeable (−4.99, 95% CI: −8.96 to −1.02, P=0.01). In the post-surgical non ESS subgroup there was no reduction in SNOT-22 score with the difference being a positive value of 0.40 (95% CI: –7.47 to 8.27) this result was also not statistically significant. It should however be noted that only one study provided data that was able to be included in the post-surgical non ESS subgroup.
Regarding clinical significance, we compared our SNOT-22 score difference to the predefined MCID score of 8.9. The overall pooled SNOT-22 score and non-surgical subgroup failed to meet this threshold. The post-surgical ESS subgroup met the predefined MCID score, suggesting that xylitol is an effective additive in nasal preparations when given to post-surgical ESS patients with sinonasal disease at reducing overall disease burden as our subgroup analysis demonstrates both a statistically and clinically significant difference when compared to normal saline.
Some patients however, may still report a clinically meaningful change in symptomology despite their SNOT-22 response not exceeding the MCID. Phillips et al. examined the MCID for SNOT-22 difference in medically managed CRS patients and determined the score to have high specificity whilst having poor sensitivity (23). This indicates that a considerable number of medically managed CRS patients may experience a clinically significant improvement despite their SNOT-22 response not meeting the predefined MCID threshold. Phillips et al. hypothesised that a disproportionate improvement across the SNOT-22 symptom domains could account for the discrepancy. They demonstrated that in a group of patients with a subthreshold MCID score whilst also reporting symptom improvement, the improvement was in the nasal symptom subdomain (15). Relating this back to our analysis, xylitol could also be beneficial in medically managed CRS patients where nasal symptoms are main contributor to their overall symptomology.
Several limitations have been identified in our review. Our meta-analysis was limited by the available literature pertaining to the use of xylitol nasal preparations in sinonasal disease with only 7 small scale RCTs identified prior to assessing for eligibility. Of which 2 unfortunately had to be excluded as they did not include the SNOT-22 score as their outcome measure, preventing a comparable and quantifiable overall assessment of effect. As result we had a total n=191 therefore making our study likely to be influenced by sampling error. It should however be noted that at the time of writing this review, 9 clinical trials looking into xylitol and sinonasal disease are currently registered on the Cochrane Central Register of Controlled Trials.
Significant heterogeneity was present between the included studies, evident in an overall I2 statistic of 85%, restricting our comparison of studies and thereby increasing the risk of confounding. This is owed to the variable methodology that exists between the included studies with distinct differences in concentrations of solution, frequency of treatment and study duration. This variability could be explained by the lack of robust evidence at present investigating the use of xylitol in sinonasal disease resulting in a yet to be determined treatment protocol.
A major contributor of bias seen across all included studies was regarding the blinding of participants to their respective intervention or control groups. This was despite measures employed by the investigators such as computer randomisation or providing the solutes in unlabelled packaging. The intervention was a sugar (xylitol) being compared against a salt (saline) and in the context of nasal irrigation, it would be reasonable to expect the participants to be able to taste a difference, therefore introducing an element of blinding error. Weissman et al. commented that 21% of their participants mentioned noticing a distinct sugary aftertaste with the xylitol irrigations (24). Hayer et al. decided to forego blinding altogether in their trial (19).
Whilst the SNOT-22 score is a well validated outcome measure of sinonasal symptoms, it is a subjective quality of life measure which depends on the patient’s specific experience of sinonasal disease. Therefore, the SNOT-22 score can be subject to a certain degree of recall bias, particularly when the scoring system requires the participant to answer 22 questions pertaining to their symptoms. To address this, an objective outcome measure would be required, Cingi et al. used rhinomanometry to measure nasal airway resistance pre and post treatment. It was the only reviewed study that incorporated an objective measure and could be considered in future studies as it would reduce recall bias.
To date, there has yet to be a systematic review conducted comparing xylitol against saline as a nasal irrigation solution. Therefore, the results of this review provide a comprehensive summary of the current body of evidence. Where previous trials have reported the SNOT-22 score we compared this against the MCID score to better meaning about the results, thereby allowing the clinician to decide if xylitol would be a suitable treatment of choice for their specific patient.
Conclusions
This review demonstrates that xylitol nasal preparations may be an effective agent of choice for the treatment of sinonasal disease in post-surgical patients who have had ESS as our pooled SNOT-22 mean score difference exceeded the predefined MCID threshold for a clinically meaningful result. The pooled SNOT-22 mean score difference in the non-surgical subgroup failed to exceed the MCID threshold, however xylitol may still be able to achieve a clinically meaningful response in this subgroup if nasal symptoms were the main contributor to their overall symptomology.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-21-45/rc
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-21-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salli K, Lehtinen MJ, Tiihonen K, et al. Xylitol's Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019;11:1813. [Crossref] [PubMed]
- Zabner J, Seiler MP, Launspach JL, et al. The osmolyte xylitol reduces the salt concentration of airway surface liquid and may enhance bacterial killing. Proc Natl Acad Sci U S A 2000;97:11614-9. [Crossref] [PubMed]
- Carothers DG, Graham SM, Jia HP, et al. Production of beta-defensin antimicrobial peptides by maxillary sinus mucosa. Am J Rhinol 2001;15:175-9. [Crossref] [PubMed]
- Teixeira Essenfelder L, Gomes AA, Miquelutti D, et al. Effect of xylitol on salivary β-glucosidase in humans. Eur J Oral Sci 2019;127:472-5. [Crossref] [PubMed]
- Salli KM, Forssten SD, Lahtinen SJ, et al. Influence of sucrose and xylitol on an early Streptococcus mutans biofilm in a dental simulator. Arch Oral Biol 2016;70:39-46. [Crossref] [PubMed]
- Staszczyk M, Jurczak A, Magacz M, et al. Effect of Polyols and Selected Dental Materials on the Ability to Create a Cariogenic Biofilm-On Children Caries-Associated Streptococcus Mutans Isolates. Int J Environ Res Public Health 2020;17:3720. [Crossref] [PubMed]
- Jain R, Lee T, Hardcastle T, et al. The in vitro effect of xylitol on chronic rhinosinusitis biofilms. Rhinology 2016;54:323-8. [Crossref] [PubMed]
- Cam B, Sari M, Midi A, et al. Xylitol treats nasal mucosa in rhinitis medicamentosa: an experimental rat model study. Eur Arch Otorhinolaryngol 2019;276:3123-30. [Crossref] [PubMed]
- Kontiokari T, Uhari M, Koskela M. Effect of xylitol on growth of nasopharyngeal bacteria in vitro. Antimicrob Agents Chemother 1995;39:1820-3. [Crossref] [PubMed]
- Liu T, Cooper T, Earnshaw J, et al. Disease burden and productivity cost of chronic rhinosinusitis patients referred to a tertiary centre in Australia. Aust J Otolaryngol 2018;1:5. [Crossref]
- Chen S, Zhou A, Emmanuel B, et al. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr Med Res Opin 2020;36:1897-911. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Rocchi MB, Sisti D, Benedetti P, et al. Critical comparison of nine different self-administered questionnaires for the evaluation of disability caused by low back pain. Eura Medicophys 2005;41:275-81. [PubMed]
- Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol 2009;34:447-54. [Crossref] [PubMed]
- Phillips KM, Hoehle LP, Caradonna DS, et al. Determinants of noticeable symptom improvement despite sub-MCID change in SNOT-22 score after treatment for chronic rhinosinusitis. Int Forum Allergy Rhinol 2019;9:508-13. [Crossref] [PubMed]
- Chowdhury NI, Mace JC, Bodner TE, et al. Does Medical Therapy Improve SinoNasal Outcomes Test-22 Domain Scores? An Analysis of Clinically Important Differences. Laryngoscope 2019;129:31-6. [Crossref] [PubMed]
- Cingi C, Birdane L, Ural A, et al. Comparison of nasal hyperosmolar xylitol and xylometazoline solutions on quality of life in patients with inferior turbinate hypertrophy secondary to nonallergic rhinitis. Int Forum Allergy Rhinol 2014;4:475-9. [Crossref] [PubMed]
- Sanchez-Gonzalez M, Rizvi SA, Torres J, et al. A Randomized Controlled Pilot Trial to Test the Efficacy of Intranasal Chlorpheniramine Maleate With Xylitol for the Treatment of Allergic Rhinitis. Cureus 2021;13:e14206. [Crossref] [PubMed]
- Hayer SD, Rabago DP, Amaza IP, et al. Effectiveness of nasal irrigation for chronic rhinosinusitis and fatigue in patients with Gulf War illness: protocol for a randomized controlled trial. Contemp Clin Trials 2015;41:219-26. [Crossref] [PubMed]
- Higgins JP, Savović J, Page MJ, et al. Assessing risk of bias in a randomized trial. Cochrane Handbook for Systematic Reviews of Interventions 2019:205-28.
- Kim DH, Kim Y, Lim IG, et al. Effect of Postoperative Xylitol Nasal Irrigation on Patients with Sinonasal Diseases. Otolaryngol Head Neck Surg 2019;160:550-5. [Crossref] [PubMed]
- Ur-Rehman S, Mushtaq Z, Zahoor T, et al. Xylitol: a review on bioproduction, application, health benefits, and related safety issues. Crit Rev Food Sci Nutr 2015;55:1514-28. [Crossref] [PubMed]
- Phillips KM, Hoehle LP, Caradonna DS, et al. Minimal clinically important difference for the 22-item Sinonasal Outcome Test in medically managed patients with chronic rhinosinusitis. Clin Otolaryngol 2018;43:1328-34. [Crossref] [PubMed]
- Weissman JD, Fernandez F, Hwang PH. Xylitol nasal irrigation in the management of chronic rhinosinusitis: a pilot study. Laryngoscope 2011;121:2468-72. Erratum in: Laryngoscope 2012;122:2611. [Crossref] [PubMed]
Cite this article as: Hui N, Yii N, Robinson D. Xylitol nasal preparations in sinonasal disease: a literature review and meta-analysis. Aust J Otolaryngol 2022;5:8.


