
Office-based unsedated bronchoscopy for the diagnosis of excessive dynamic airways collapse
Introduction
Excessive dynamic airway collapse (EDAC) is an increasingly recognised condition that can present with symptoms of obstructive airway disease. It is defined as the pathological narrowing of the trachea or main bronchi by >50% on expiration as a result of the invagination of a slack posterior tracheobronchial membrane (1). It is classified under the umbrella term of excessive central airway collapse (ECAC), along with tracheobronchomalacia (TBM), a closely related condition that occurs as a result of instability or weakness of the airway cartilage (1). The true prevalence of these disorders is unknown, partly due to variations in definition and presentation, and also due to difficulty in diagnosis.
While pathophysiology of the two conditions differs, clinical presentations and management principles are similar (2). EDAC is often diagnosed following investigation of dyspnoea, wheeze, chronic cough, or presumed asthma and COPD which are refractory to bronchodilators and inhaled corticosteroids (3). Typical symptoms include a seal barking cough, shortness of breath on exertion, difficulty clearing secretions, and exertional or coughing syncope (1). This cohort of patients is often referred to the respiratory physician or laryngologist for the consideration of obstructive respiratory diseases or functional laryngeal disorders such as inducible laryngeal obstruction (ILO) or vocal cord dysfunction (VCD), and as such diagnosis of EDAC is important as its management differs from these conditions. Medical treatment involves pulmonary rehabilitation, breathing techniques, and CPAP for pneumatic stenting. In severe cases, short-term airway stabilisation with airway stents can provide temporary benefit and determine the effectiveness of definitive surgical airway stabilisation (4).
Dynamic flexible bronchoscopy is considered the gold standard for diagnosis of EDAC, as it allows objective real-time evaluation of the airway in inspiratory and expiratory phases (5). Low-dose dynamic airway CT has been validated as a reliable diagnostic tool either as a non-invasive alternative to bronchoscopy or in conjunction with bronchoscopy, and can quantify the degree of expiratory collapse of the larger airways (6,7). However, dynamic flexible bronchoscopy has traditionally been performed under sedation which requires post-procedure monitoring and has related sedation risks and facility costs. Dynamic CT confers an additional radiation risk in patients that are often already extensively investigated with multiple previous CTs.
This retrospective case series of 11 patients describes the use of office-based local anaesthetic transnasal tracheo-bronchoscopy as an underutilised approach for the diagnosis of EDAC, which avoids sedation and radiation exposure risks, and allows for dynamic patient participation during diagnosis and allows for real-time biofeedback.
We present the following article in accordance with the STARD reporting checklist (available at https://dx.doi.org/10.21037/ajo-20-90).
Methods
This retrospective case series follows eleven consecutive patients referred to a laryngologist’s private non-hospital-based clinic from 2018 to 2019 for further investigation of respiratory symptoms, not explained by a complete respiratory work-up. In all cases, a diagnosis of ILO or airway stenosis was queried by the referring physician, and no functional or structural abnormality of the glottic or subglottic larynx was seen on flexible nasendoscopy. All 11 patients underwent transnasal video bronchoscopy. With the unsedated patient seated in a chair, local anaesthetic was applied to the upper airway in the form of a 1 mL of nasal spray of 5% lignocaine and 5% phenylephrine. In addition, 2 mL of 5% lignocaine was also applied to the airway via a trans-tracheal injection. The 3.6 mm diameter, flexible video-nasopharyngoscope (Xion® medical, GMBH) was then passed transnasally, down to the level of the secondary bronchi. In this way the upper aero-digestive tract was directly visualised. The fixed and dynamic anatomy of the supraglottis, glottis, subglottis, trachea and primary bronchi down to the secondary bronchi was evaluated. From an EDAC perspective, particular attention was paid to membranous and/or cartilaginous integrity of the tracheobronchial tree.
In patients with a history of exertional dyspnoea but in whom the initial passage of the scope was normal, provocation was trialled. In these cases, patients who had already had topical anaesthesia, were instructed to walk briskly for approximately 50m or up and down two flights of stairs until symptomatic, before returning to the examination chair for immediate endoscopy. In others, symptoms were evident during endoscopy, on minor exertion in the chair or able to be mimicked. During the assessment with the scope in-situ, patients were also provided visual feedback of their condition and repatterning breathing techniques were demonstrated to determine stimulability for change from an EDAC ‘posture’ to minimal tracheal narrowing on expiration. These techniques included instruction to consciously slow expiration and decrease effort (i.e., reduce expiratory flow rates and accessory muscle hyperfunction), minimise expiratory noise, and repattern respiratory rhythms. Patients were observed in the office waiting room for 15 minutes after their procedure, for any clinical symptoms of respiratory complications or adverse reactions to the local anaesthetic agents, before being able to leave the clinic unaccompanied. In keeping with the clinic’s routine in-office naso-laryngo-pharyngoscopy and trans-nasal-oesophagoscopy procedures, continuous monitoring was not utilised during or post-procedure. Supplemental oxygen, bag-valve-mask resuscitators, cricothyroidotomy kits and adrenaline were all available in case of any deterioration, but were not required in any of the cases.
Video recording of the bronchoscopy in entirety was performed and reviewed post procedure, for accurate analysis of airway diameter and nature and degree of airway collapse. For further diagnostic accuracy, de-identified videos of five separate unsedated bronchoscopies, of patients both with and without a diagnosis of EDAC, were provided to two respiratory physicians for review. Two of the five videos were repeated, to make a total of seven videos reviewed, to assess intrarater reliability.
The study protocol was approved by the Institutional Review Board of Monash Health (NHMRC approval HREC QA/66900/MonH-2020-223277). Due to the retrospective nature of this study, the need for informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Patients’ history, examination, investigations, management and follow up were extracted from patient files. Descriptive values regarding patient demographics, and information regarding procedure tolerance, diagnosis of EDAC, or alternate diagnoses, were extrapolated into table form. Raw numbers were summarised, and the intra- and inter-rater reliability of two independent reviewers of bronchoscopy videos were analysed.
A literature review regarding current diagnostic methods for EDAC was performed.
Results
Over a two-year period, unsedated trans-nasal bronchoscopy was performed in 11 patients suspected of having a diagnosis of EDAC. Age ranged from 16 to 81-year-old, and ten of the 11 patients were female (Table 1). The majority of these patients were referred by an allergist or respiratory physician, questioning the possible diagnosis of paradoxical vocal fold movement (PVFM) or VCD as an explanation for their various presenting symptoms such as chronic cough, exertional dyspnoea, wheeze and dysphonia.
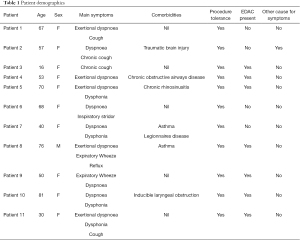
Full table
The procedure was well tolerated by all 11 patients, with minimal reported discomfort. There were no adverse events of laryngospasm or acute dyspnoea, and all patients left the clinic after an uncomplicated 15 minute monitoring period. Satisfactory views of the larger airways, extending down to the secondary bronchi, were successfully obtained in all 11 patients.
A diagnosis of EDAC was made in 7 of 11 patients based on the presence of greater than 50% dynamic expiratory collapse/bulging of the posterior membrane at rest or post exertion. Figures 1,2 show views of the larger airways, with invagination of the posterior membrane. In all cases, the degree of EDAC was mild to moderate and no request for forced expiration was deemed necessary. All patients diagnosed with EDAC were introduced to therapeutic breathing techniques and all seven were able to modify the extent or presence of EDAC during the endoscopic evaluation.

On review of de-identified videos of unsedated bronchoscopies, two respiratory physicians agreed with the diagnosis of EDAC in 70% of cases, with an inter-rater reliability of 80%. Intrarater reliability was 100%, with both clinicians coming to the same diagnosis when showed the same video twice. In the one bronchoscopy where an in-office diagnosis of EDAC was made, but both blinded clinicians disagreed with the diagnosis of EDAC, there was further uncertainty in diagnosis. One diagnosis of mild bronchomalacia was offered, while the other diagnosis was of an incidental finding of tracheobronchopathia osteochondroplastica.
Discussion
Excessive dynamic airway collapse should be considered as a differential diagnosis in patients presenting with nonspecific respiratory or upper airway symptoms, or in whom routine medical management has been unsuccessful. EDAC commonly occurs in patients with chronic airway inflammation (8), and symptoms, including shortness of breath on exertion, barking cough, wheeze/expiratory stridor, inability to clear secretions and subsequent recurrent respiratory infections, can mimic many other common respiratory diseases. Park et al. found an incidence of EDAC of 22% in all patients undergoing bronchoscopy with a pre-procedural diagnosis of COPD.(9) Additionally, 14% of patients with chronic cough and 13% of patients with known lung disease had evidence of ECAC found on dynamic bronchoscopy (1). Diagnosis of EDAC is traditionally based on either bronchoscopic evaluation or dynamic cross-sectional imaging; many studies considering an airway narrowing of >50% as diagnostic for EDAC (5,10,11), with some studies using a tighter threshold of >70% airway narrowing (4).
Pulmonary function testing has value in excluding obstructive or restrictive lung diseases as a primary cause of symptoms, but is neither sensitive nor specific for EDAC (12). Findings may indicate airflow limitation, with low maximum forced expiratory flow, notched expiratory loop, biphasic morphology, and flow oscillations (1,13). Majid et al. evaluated pulmonary function test results of patients with symptomatic moderate to severe TBM/EDAC and found varying patterns of obstructive (44.4%), restrictive (17.8%), and mixed (16.7%) defects, with normal PFT results found in 21.1% of this cohort.
Dynamic multidetector computerised tomography (MDCT) imaging of the larger airways has been validated as a safe and accurate non-invasive diagnostic method (6,7,14). Patients are instructed to perform respiratory manoeuvres, and cross-sectional dynamic images of the trachea and bronchi are captured in real time and measured to diagnose and quantify EDAC. Modalities can include paired end-inspiratory and dynamic expiratory MDCT, cine-CT during coughing, and paired end-inspiratory and end-expiratory MDCT for patients unable to cooperate with dynamic breathing instructions (14). Lee et al. found dynamic CT had 97% accuracy when compared to bronchoscopy for the evaluation of TBM/EDAC (15). Additional reports in the literature have diagnosed EDAC as an incidental finding on nondynamic CT (16). Taherian et al. evaluated computational fluid dynamics (CFD) results of a patient with EDAC, analysing CFD simulations of CT-based models pre- and post-airway stenting with promising results (17). Radiological evaluation can be seen as fallible, however, with two studies showing CT evidence of collapse greater than 50% in up to 78% of asymptomatic patients (15,18).
Dynamic flexible fibreoptic bronchoscopy (FFB) with direct visualisation of the larger airways is considered the gold standard for the diagnosis of EDAC, as it allows real-time evaluation of dynamic airway properties and direct airway mucosal visualisation (4,5). Most patients undergo transnasal or transoral flexible endoscopy under varying levels of sedation based on clinician preference. Narrow, flexible video bronchoscopes, commonly with a 4.9 mm outer diameter and 2.0 mm working channels are favoured over larger therapeutic bronchoscopes, to avoid unintentional stenting effects on the airway (1,4). Evaluation of the airway for collapse includes the proximal trachea at the level of the cricoid, mid-trachea 5cm proximal to the carina, distal trachea 2cm proximal to the carina, right mainstem bronchus at the right tracheobronchial angle, bronchus intermedius, and left main bronchus at the left tracheobronchial angle. Patients are asked to inspire deeply and rapidly exhale, with analysis of airways during these forced manoeuvres and at normal tidal breathing. Manoeuvers are repeated three times, and images are captured and measured for degree of collapse present (1,4).
The routine use of sedation in diagnostic bronchoscopy has been debated recently within the literature. The British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults currently recommends intravenous sedation to be offered to all patients, provided there are no contraindications (19). However, studies have reported up to half of major life-threatening complications of FFB have been sedation-related (20,21), with complications including hypotension, respiratory depression, respiratory arrest, and laryngospasm (22). The use of sedation requires constant cardiorespiratory monitoring with ECG and pulse oximetry, and requires recovery and monitoring post procedure. Unsedated bronchoscopy is a viable alternative; studies have reported diagnostic FFB being performed adequately without the use of sedation, and a study in 1991 showed up to 49% of all bronchoscopies were performed without sedation (23-25). Pearce reported that 95 out of 100 patients premedicated with atropine 0.6 mg, 2 benzocaine lozenges, nasal spray of cocaine 5% and pharyngeal spray of lignocaine 4%, found the procedure ‘no bother’ or ‘a bit uncomfortable’ (24). If well tolerated, unsedated bronchoscopy is advantageous over sedated bronchoscopy due to the avoidance of sedation-related adverse events, and does not require continuous monitoring and post-procedure observation. Some studies within otolaryngology journals have described in-office unsedated transnasal bronchoscopy as a well-tolerated, accurate method for the diagnosis of certain other tracheobronchial pathologies (26,27).
Successful topicalisation of the airway, in particular the larynx and subglottis, is critical to ensure awake fibreoptic bronchoscopy is well tolerated. The nasal airway is commonly sprayed with a combination of nasal decongestant and local anaesthetic, prior to introduction of transnasal fibreoptic bronchoscope. Lignocaine is the most commonly utilised local anaesthetic agent, that can be applied to the airway either through a percutaneous, transtracheal approach, where a needle is passed through the skin to the cricothyroid membrane, through a transnasal catheter, or by transoral aspiration of lignocaine while lying supine (26,28). Lignocaine toxicity is rare, but at doses over 4.5 mg/kg, sequelae can include hypotension, bradycardia, cardiac/respiratory arrest, and CNS depression (29). Varying volumes of lignocaine, in concentrations between 1% and 5% are commonly used, but doses required for successful anaesthesia of the airway rarely exceed toxic doses of lignocaine, with Morris et al. finding that no more of 3 mL of 4% lignocaine was required for procedural tolerance (26).
Management of EDAC ranges from supportive treatment to surgical management in severe cases. Mild symptomatic cases may benefit from optimisation of other medical conditions that may contribute to symptoms, including management of concurrent gastro-oesophageal reflux disease/laryngopharyngeal reflux, obstructive sleep apnoea, asthma, or COPD (4,12). Additionally, respiratory retraining, pulmonary rehabilitation and weight loss programs show promise (4), but more studies are needed to evaluate the aetiology and nature of EDAC, whether it represents a form of dysfunctional breathing, and the effectiveness of behavioural interventions. Continuous positive airway pressure (CPAP) is a non-invasive ventilation method that can be used for pneumatic stenting, and may help respiratory symptoms, secretion clearance and exercise tolerance to some degree, however there are no studies showing its long-term effects (30,31). Severe symptomatic EDAC, with evidence of luminal reduction >90%, should be evaluated for stenting and surgical repair (10,12). Stent trials with silicone or metal stents have been demonstrated to improve quality of life and dyspnoea scores (32), and are able to determine the degree to which airway collapsibility contributes to a patient’s symptoms, and suitability for surgical intervention. The most common surgical intervention for EDAC is tracheobronchoplasty, which reinforces the posterior membrane with mesh and tightens the tracheal rings (33). Studies have shown significant improvement in pulmonary function testing results, 6-minute walk tests and performance status following tracheobronchoplasty for severe EDAC (33,34). Other surgical options include tracheostomy, which provides airway stenting, while allowing for positive pressure ventilation (1).
The results of this case series show unsedated, office-based bronchoscopy is a viable and well tolerated method for the investigation and diagnosis of EDAC, with many advantages over existing evaluation tools for EDAC. The ability to perform bronchoscopy without sedation allows for maximal patient engagement in order to monitor dynamic changes within the airway, and in the case of a negative examination provides the option for patients to be physically exerted prior to re-examination, in order to elucidate exertional symptoms. It also allows for clinicians to provide videographic feedback and to commence education regarding therapeutic breathing techniques. Additionally, the absence of any immediate or delayed cardiac or respiratory complications validate this as a technique that can be performed in an office-based setting without continuous monitoring or support, over sedated bronchoscopy in a procedure room as it mitigates the risk of sedation-related adverse events and delayed wait-list times. The ability to directly visualise the airway and lack of radiation exposure make it a preferred option over dynamic multi-detector CT. This is a particularly useful and readily available diagnostic method that allows for prompt, in-office initiation of management of therapeutic breathing techniques and/or CPAP in most cases.
Limitations to this study include the retrospective study design, small sample size, and a heterogeneous patient demographic. Additionally, there was no direct comparison between unsedated bronchoscopy, sedated bronchoscopy or CT. It does, however, provide the impetus for further research into an under-recognised clinical population, and an under-utilised diagnostic technique. A prospective study comparing patient tolerance and engagement levels, degree of visualisation, and complication rates between sedated and unsedated bronchoscopy may be of use in the future to further validate unsedated bronchoscopy as a safe, readily available and effective method in the diagnosis of EDAC. While EDAC has seen recent advancements within the medical literature, it is still a poorly understood condition. The discrepancies between diagnostic criteria in different studies, and the volume of asymptomatic patients satisfying diagnostic criteria for EDAC, raise the question that EDAC may be a behaviourally-mediated condition, and may represent a form of dysfunctional breathing. Future studies evaluating the criteria for diagnosis and responsiveness to respiratory retraining techniques as a first-line management strategy may shed more light on the true aetiology and nature of the condition.
Conclusions
Unsedated transnasal bronchoscopy was well-tolerated in all 11 patients of this case series, allowing evaluation of the upper and middle airways in an office-based setting without the need for specialised equipment or continuous monitoring, and without sedation-related risks. Utilising this technique allowed for diagnosis of EDAC in 7 out of 11 patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://dx.doi.org/10.21037/ajo-20-90
Data Sharing Statement: Available at https://dx.doi.org/10.21037/ajo-20-90
Peer Review File: Available at https://dx.doi.org/10.21037/ajo-20-90
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/ajo-20-90). PP serves as an unpaid editorial board member of the Australian Journal of Otolaryngology from Jan 2019 to Dec 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of Monash Health (NHMRC approval HREC QA/66900/MonH-2020-223277). Due to the retrospective nature of this study, the need for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hammond K, Ghori UK, Musani AI. Tracheobronchomalacia and excessive dynamic airway collapse. Clin Chest Med 2018;39:223-8. [Crossref] [PubMed]
- Murgu S, Colt H. Tracheobronchomalacia and excessive dynamic airway collapse. Clin Chest Med 2013;34:527-55. [Crossref] [PubMed]
- Kalra A, Abouzgheib W, Gajera M, et al. Excessive dynamic airway collapse for the internist: new nomenclature or different entity? Postgrad Med J 2011;87:482-6. [Crossref] [PubMed]
- Kheir F, Majid A, editors. Tracheobronchomalacia and Excessive Dynamic Airway Collapse: Medical and Surgical Treatment. Seminars in respiratory and critical care medicine; 2018: Thieme Medical Publishers.
- Majid A, Gaurav K, Sanchez JM, et al. Evaluation of tracheobronchomalacia by dynamic flexible bronchoscopy. A pilot study. Ann Am Thorac Soc 2014;11:951-5. [Crossref] [PubMed]
- Joosten S, MacDonald M, Lau KK, et al. Excessive dynamic airway collapse co-morbid with COPD diagnosed using 320-slice dynamic CT scanning technology. Thorax 2012;67:95-6. [Crossref] [PubMed]
- Zhang J, Hasegawa I, Feller-Kopman D, et al. Dynamic expiratory volumetric CT imaging of the central airways: comparison of standard-dose and low-dose techniques. Acad Radiol 2003;10:719-24. [Crossref] [PubMed]
- Ridge CA, O'Donnell CR, Lee EY, et al. Tracheobronchomalacia: current concepts and controversies. J Thorac Imaging 2011;26:278-89. [Crossref] [PubMed]
- Park JG, Edell E. Dynamic airway collapse. Different from tracheomalacia. Rev Port Pneumol 2005;11:600. [PubMed]
- Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006;11:388-406. [Crossref] [PubMed]
- Carden KA, Boiselle PM, Waltz DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest 2005;127:984-1005. [Crossref] [PubMed]
- Choo EM, Seaman JC, Musani AI. Tracheomalacia/tracheobronchomalacia and hyperdynamic airway collapse. Immunol Allergy Clin North Am 2013;33:23-34. [Crossref] [PubMed]
- Majid A, Sosa AF, Ernst A, et al. Pulmonary function and flow-volume loop patterns in patients with tracheobronchomalacia. Respir Care 2013;58:1521-6. [Crossref] [PubMed]
- Lee EY, Litmanovich D, Boiselle PM. Multidetector CT evaluation of tracheobronchomalacia. Radiol Clin North Am 2009;47:261-9. [Crossref] [PubMed]
- Boiselle PM, O'Donnell CR, Bankier AA, et al. Tracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CT. Radiology 2009;252:255-62. [Crossref] [PubMed]
- Harada Y, Kondo T. Excessive dynamic airway collapse detected using nondynamic CT. Intern Med 2016;55:1477-9. [Crossref] [PubMed]
- Taherian S, Rahai H, Gomez B, et al. Computational fluid dynamics evaluation of excessive dynamic airway collapse. Clin Biomech (Bristol, Avon) 2017;50:145-53. [Crossref] [PubMed]
- Stern EJ, Graham C, Webb W, et al. Normal trachea during forced expiration: dynamic CT measurements. Radiology 1993;187:27-31. [Crossref] [PubMed]
- Pickles J, Jeffrey M, Datta A, et al. Is preparation for bronchoscopy optimal? Eur Respir J 2003;22:203-6. [Crossref] [PubMed]
- Putinati S, Ballerin L, Corbetta L, et al. Patient satisfaction with conscious sedation for bronchoscopy. Chest 1999;115:1437-40. [Crossref] [PubMed]
- Reed AP. Preparation of the patient for awake flexible fiberoptic bronchoscopy. Chest 1992;101:244-53. [Crossref] [PubMed]
- Credle WF Jr, Smiddy JF, Elliott RC. Complications of fiberoptic bronchoscopy. Am Rev Respir Dis 1974;109:67-72. [PubMed]
- Colt HG, Morris JF. Fiberoptic bronchoscopy without premedication: a retrospective study. Chest 1990;98:1327-30. [Crossref] [PubMed]
- Pearce SJ. Fibreoptic bronchoscopy: is sedation necessary? Br Med J 1980;281:779. [Crossref] [PubMed]
- Prakash UB, Offord KP, Stubbs SE. Bronchoscopy in North America: the ACCP survey. Chest 1991;100:1668-75. [Crossref] [PubMed]
- Morris LG, Zeitler DM, Amin MR. Unsedated flexible fiberoptic bronchoscopy in the resident clinic: technique and patient satisfaction. Laryngoscope 2007;117:1159-62. [Crossref] [PubMed]
- Shah MD. MPhila III MMJ. Office-based laryngeal procedures. Office Procedures in Laryngology An Issue of Otolaryngologic Clinics-E-Book 2012;46:75.
- Mainland P-A, Kong AS, Chung DC, et al. Absorption of lidocaine during aspiration anesthesia of the airway. J Clin Anesth 2001;13:440-6. [Crossref] [PubMed]
- Torp KD, Metheny E, Simon LV. Lidocaine toxicity. StatPearls [Internet] 2020.
- Patout M, Mylott L, Kent R, et al. Trial of portable continuous positive airway pressure for the management of tracheobronchomalacia. Am J Respir Crit Care Med 2016;193:e57-e.
- Ferguson GT, Benoist J. Nasal Continuous Positive Airway Pressure in the Treatment of Tracheobronchomalacia. Am Rev Respir Dis 1993;147:457-61. [Crossref] [PubMed]
- Ernst A, Majid A, Feller-Kopman D, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest 2007;132:609-16. [Crossref] [PubMed]
- Wright CD, Grillo HC, Hammoud ZT, et al. Tracheoplasty for expiratory collapse of central airways. Ann Thorac Surg 2005;80:259-66. [Crossref] [PubMed]
- Majid A, Guerrero J, Gangadharan S, et al. Tracheobronchoplasty for severe tracheobronchomalacia: a prospective outcome analysis. Chest 2008;134:801-7. [Crossref] [PubMed]
Cite this article as: Chia C, Phyland DJ, Steinfort D, Leong TL, Paddle P. Office-based unsedated bronchoscopy for the diagnosis of excessive dynamic airways collapse. Aust J Otolaryngol 2021;4:29.



