
The role of tonsillar actinomycosis in adult patients
Introduction
Tonsillectomy is one of the most commonly performed surgical procedures. There are many indications for the surgery, and these vary for children and adults. Routine histological testing is done in adult tonsillectomy specimens in certain centres, however this may not be a viable option in resource limited centres (1). A common finding on the tonsillectomy histology is the presence of the bacteria actinomycosis.
The objective of the study was to establish the incidence of actinomycosis in the tonsils of adults undergoing tonsillectomy and to evaluate its role in clinical tonsillar disease.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-19-38).
Background
Actinomycosis is caused by gram-positive, non-acid fast, anaerobic, branching, filamentous bacteria which result in chronic suppurative inflammation of the organs involved. The organism is a commensal in the human oral cavity (2). Actinomyces colonization of tonsillar crypts has been described in 7–35% of tonsillectomy specimens (3,4). Aydin et al. and Toh et al. found actinomycosis more common in adults than in children (5,6).
It has been suggested that tonsillar actinomycosis may be an aetiological factor in the development of tonsillar hypertrophy (5,6). This finding has not been consistent. A paediatric study by van Lierop et al. examined 344 tonsils and found no tissue reaction due to Actinomyces colonies and hence reported that there was no correlation between tonsillar actinomycosis and recurrent tonsillitis in their population (1). Melgarejo et al. found no statistical association between Actinomyces and recurrent adenotonsillitis or adenotonsillar hypertrophy (7).
Methods
Study design
This study is a retrospective record review, for period 1 July 2005 to 30 June 2015.
Study location
The study was conducted in the clinical units of the University of the Witwatersrand’s Department of Otorhinolaryngology (ENT). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained in March 2016 from the Human research ethics committee University of the Witwatersrand (registration number M151140) and individual consent for this retrospective analysis was waived.
Study population
The study population included all patients older than 18 years of age, who had a tonsillectomy performed. All patients undergoing tonsillectomies had histological analysis.
Inclusion criteria: all adult patients who had a tonsillectomy performed and for whom histopathological results were available on the National Health Laboratory Services (NHLS) database.
Exclusion criteria: inadequate data.
Data collection
Patients were identified from the ENT Operating Theatre Register. The patient information was used to obtain the histopathology reports from the NHLS database.
Data recorded included
- Hospital registration number;
- Age;
- Date of surgery;
- Histology report;
- Patients were divided into two groups:
- The sample group consisted of those with actinomycoses identified on histology;
- The control group were patients without actinomycoses;
- The histology reports were reviewed by the authors and a histopathologist.
The following details were recorded:
- The presence of actinomycoses in the tonsillectomy specimens
- The site of the bacteria was also reviewed and deemed to be either in the tonsillar:
- Stroma;
- Crypts;
- Both tonsillar stroma and crypts.
- The association between the presence of actinomycoses and tonsillar hyperplasia in the specimens was evaluated using a two by two contingency table.
Fischer’s exact test was performed using GraphPad.
- Ethics approval was obtained in March 2016 from the Ethics Committee, University of the Witwatersrand. Certificate number M151140.
Limitation: small number of patients with actinomycosis.
Results
There were 638 specimen reports reviewed, comprising 319 patients. Actinomycosis was documented in 74 specimens.
The frequency of actinomycosis in the tonsils of adults undergoing tonsillectomy was 11.6% in our study population.
Ninety-eight percent of patients with actinomycosis had reactive tonsillar hypertrophy as compared to the control group (No Actinomycoses) in whom 77% of patients had tonsillar hypertrophy. This difference was significant (P=0.002).
Patient demographics
In the study sample the patient ages ranged from 18 to 77 years old, with a mean age of 35 years and a standard deviation of 12 years. In the cohort with actinomycosis the average age was 35 years with a standard deviation of 11 years. There were 23 females and 14 males.
Histopathology
The reports revealed that reactive lymphoid hyperplasia was the most common (77%) condition encountered amongst our patients. The next most commonly described condition was actinomycosis which was documented in 74 (14%) specimens.
The majority of specimens with actinomycoses identified on histopathology, demonstrated reactive tonsillar hyperplasia 36/37 (97%). In the control group only 77% had reactive tonsil hyperplasia.
Actinomycoses infection was significant in the development of reactive tonsil hyperplasia (P=0.002) (see Table 1).
Table 1
| Variables | Actinomycoses group | Control group |
|---|---|---|
| Number of patients | 37 | 282 |
| Gender | ||
| Female | 23 | 198 |
| Male | 14 | 84 |
| F:M ratio | 1.6:1 | 2.4:1 |
| Reactive tonsil hyperplasia | 36/37 (97%) | 218/282 (77%) |
Actinomycoses infection was significant in the development of reactive tonsil hyperplasia (P=0.002).
The most common site involved was the stroma; 58 specimens had Actinomycoses in the stroma. Sixteen specimens had Actinomycoses in the tonsillar crypts (Figure 1). In our sample no patients were reported as having Actinomycoses in both the stroma and the crypts.
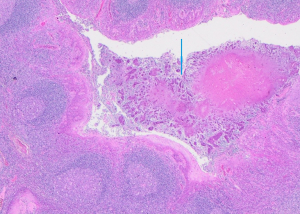
Discussion
The presence of actinomycosis infection in tonsillectomy specimens is well described. The incidence is variable and ranges between 0.8% and 41% (8). In this study the incidence was 11.6%. The reasons for this variation include differences in the sectioning and staining techniques employed by different laboratories; the ages of the patients studied and discrepancies in the indications for tonsillectomy.
Hematoxylin & eosin (H&E) staining is useful in the detection of Actinomyces colonies. There are more sophisticated techniques including polymerase chase reaction-based oligonucleotide-deoxyribonucleic acid hybridization technique and Restriction fragment length polymorphism analysis of amplified small subunit ribosomal Ribonucleic acid genes which can improve the yield (9,10). In 1991 Pransky et al. suggested that core tissue analysis be used to assess tonsillar infection as this may be shielded from the effect of antimicrobials, and anaerobic organisms are more likely to survive in the oxygen depleted environment of the tonsillar crypts (11). They found that microbiological investigations are only successful in determining the presence of Actinomycetes in less than half of the cases; and that histological examination of the core tissues of the tonsils will more accurately detect the presence of these organisms (11).
Actinomyces are commonly found in the oral cavity where they are commensals. Their role in the development of tonsillar disease has not been firmly established. Numerous series have been published to try and establish the relationship between tonsillar actinomycosis and clinical tonsillar disease (6,9-13). In 1910 Lord isolated Actinomyces species from the tonsillar crypts and suggested that hypertrophy of the tonsils is the result of some toxin produced by the micro-organisms (12). This has however never been proven.
Ashraf et al. studied 204 patients who underwent tonsillectomies. In their study cohort indications were recurrent tonsillitis in 81% and obstructive sleep apnea (OSA) in 19%. They documented actinomycosis in 41% of their patients; with a higher incidence in their adult population. They surmised that Actinomyces colonization was more prevalent in patients with recurrent tonsillitis than in those with sleep disordered breathing. They concluded that the presence of Actinomyces does not indicate any active disease (8).
Bhargava et al. reviewed 302 patients. In their group the indications for surgery were similar but the proportions were different in that only 61% had recurrent tonsillitis and 39% had OSA. They found a positive correlation between actinomycosis of the tonsils and tonsillar hypertrophy with actinomycosis present in 56.8% of patients with tonsillar hypertrophy compared to 10.3% of patients with recurrent tonsillitis (14). The findings of Rebechi et al. were similar. They evaluated the routine histopathology of 281 patients who underwent tonsillectomy with 63% having had recurrent tonsillitis and 37% OSA. They found evidence of chronic tonsillitis with colonies of Actinomyces in 9.6% of their patients (15). The discrepancy between Ashraf et al. (8), and Bhargava et al. (14) and Rebechi et al. (15) may be due to the disproportionate numbers of recurrent tonsillitis and OSA in the respective studies.
Conclusions
The frequency of actinomycosis in the tonsils of adults undergoing tonsillectomy was 11.6% in our study population. This is slightly higher than comparative studies.
Ninety-eight percent of patients with actinomycosis had reactive tonsillar hypertrophy as compared to the control group (No Actinomycoses) in whom 77% of patients had tonsillar hypertrophy. This difference was significant (P=0.002).
Based on our study it can be said that when actinomycosis is present, there is almost always lymphoid hyperplasia as well, implying a commonality in the factors that underlie these conditions.
Acknowledgments
The authors would like to thank Dr. F. Mahomed for the histology picture (Figure 1).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-19-38
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ajo-19-38
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-19-38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained in March 2016 from the Human research ethics committee University of the Witwatersrand (registration number M151140) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Lierop AC, Prescott CA, Sinclair-Smith CC. An investigation of the significance of Actinomycosis in tonsil disease. Int J Pediatr Otorhinolaryngol 2007;71:1883-8. [Crossref] [PubMed]
- Schwartz HC, Wilson MC. Cervicofacial actinomycosis following orthognathic surgery: report of 2 cases. J Oral Maxillofac Surg 2001;59:447-9. [Crossref] [PubMed]
- Soler Sendra A, Subirana Pozo FX, Consola Maroto B, et al. Tonsillar actinomycosis manifested as expectorated debris. Acta Otorrinolaringol Esp 2009;60:372-4. [Crossref] [PubMed]
- Assimakopoulos D, Vafiadis M, Askitis P, et al. The incidence of Actinomyces israeli colonization in tonsillar tissue. A histopathological study. Rev Stomatol Chir Maxillofac 1992;93:122-6. [PubMed]
- Aydin A, Erkiliç S, Bayazit YA, et al. Relation between actinomycosis and histopathological and clinical features of the palatine tonsils: a comparative study between adult and pediatric patients. Rev Laryngol Otol Rhinol (Bord) 2005;126:95-8. [PubMed]
- Toh ST, Yuen HW, Goh YH. Actinomycetes colonization of tonsils: a comparative study between patients with and without recurrent tonsillitis. J Laryngol Otol 2007;121:775-8. [Crossref] [PubMed]
- Melgarejo Moreno P, Hellin Meseguer D, Marco Garrido A, et al. A correlation between age and Actinomyces in the adenotonsillar tissue of children. B-ENT 2006;2:95-7. [PubMed]
- Ashraf MJ, Azarpira N, Khademi B, et al. Relation between Actinomycosis and Histopathological and Clinical Features of the Palatine Tonsils: An Iranian Experience. Iran Red Crescent Med J 2011;13:499-502. [PubMed]
- Tang G, Samaranayake LP, Yip HK, et al. Direct detection of Actinomyces spp. from infected root canals in a Chinese population: a study using PCR-based, oligonucleotide-DNA hybridization technique. J Dent 2003;31:559-68. [Crossref] [PubMed]
- Stubbs SLJ, Hall V, Talbot P, et al. Identification of Bacteroides and Actinomyces by 16S rDNA PCR-restriction fragment length polymorphism. Anaerobe 2000;6:121-2. [Crossref]
- Pransky SM, Feldman JI, Kearns DB, et al. Actinomycosis in obstructive tonsillar hypertrophy and recurrent tonsillitis. Arch Otolaryngol Head Neck Surg 1991;117:883-5. [Crossref] [PubMed]
- Lord FT. The etiology of actinomycetes. The presence of actinomycetes in the contents of carious teeth and tonsillar crypts of patients with actinomycosis. JAMA 1910;55:1261-3. [Crossref]
- Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist 2014;7:183-97. [PubMed]
- Bhargava D, Bhusnurmath B, Sundaram KR, et al. Tonsillar actinomycosis: a clinicopathological study. Acta Trop 2001;80:163-8. [Crossref] [PubMed]
- Rebechi G, Pontes TE, Braga EL, et al. Are histologic studies of adenotonsillectomy really necessary? Int Arch Otorhinolaryngol 2013;17:387-9. [Crossref] [PubMed]
Cite this article as: Hari KR, Maharaj S, Motakef S, Essa R. The role of tonsillar actinomycosis in adult patients. Aust J Otolaryngol 2020;3:30.


