
Assessment of macroglossia as a cause of failed continuous positive airway pressure adherence
Introduction
Continuous positive airway pressure (CPAP) is considered first line therapy for treatment of symptomatic adult patients with obstructive sleep apnoea (OSA) regardless of severity (1). Treatment failure defined as less than 4 hours of usage per night or outright refusal of therapy may occur in 29–83% of patients (2). This is commonly due to inability to adhere to CPAP. Cited reasons for failure include treatment misconceptions, dry mouth, nasal stuffiness/rhinorrhoea, claustrophobia, bloating/discomfort and concurrent comorbidities like depression or cardiovascular disease (3,4).
Obstructing anatomy may contribute to elevated CPAP pressures, which in turn are associated with device intolerance and non-adherence (5). Upper airway tissues can contribute to obstruction at the nasal, velopharyngeal, oropharyngeal, tongue or epiglottic levels (6). The tongue is an important anatomical target for salvage treatment, and there is a known association between OSA and macroglossia (7,8). The effect of macroglossia is exacerbated by obesity (9), increased mandibular plane-hyoid distance (10) and craniofacial abnormalities (7).
Macroglossia is a significant contributing factor to upper airway resistance in OSA (11). Studies utilising magnetic resonance imaging (MRI) have demonstrated a higher incidence of macroglossia in patients with OSA compared with controls (8,12), and it is a factor in the nadir oxygen saturation during polysomnography (PSG) (13). Various authors have employed three-dimensional airway reconstruction computer tomography (CT) to assess airway parameters (14,15). There have been no published studies specifically investigating the relationship between CPAP adherence and tongue size using objective CT measures such as tongue surface area (SA).
We hypothesize that patients with OSA who are non-adherent to CPAP have a larger tongue volume than those who have been successfully stabilized on CPAP using a surrogate marker of tongue volume, tongue SA. Our secondary hypothesis is that both CPAP adherent and CPAP non-adherent patients with OSA will have larger tongue SA than adults without OSA.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ajo-20-23).
Methods
Approval was obtained from the University of Wollongong Human Research and Ethics Committee (HE11/468) and written informed consent was obtained from patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Prospective recruitment of patients undergoing CT scans for OSA between the periods of 2011 and 2016 was undertaken. This cohort was derived from multiple different metropolitan and regional areas of Australia.
Power analysis, inclusion and exclusion criteria
Power analysis based on a previous study on tongue volume (9) demonstrated that a sample size of 20 was required to detect a meaningful difference in tongue volume (15,000 mm3) between OSA patients and normal patients (assuming a standard deviation of 15,000 mm3, using a two-tailed t-test with an alpha of 0.05) with 80% power. Given that there was no data available on tongue SA of OSA patients that are non-adherent to CPAP, we aimed to recruit at least 40 OSA patients and 20 non-OSA overall. Patients were excluded if they had severe psychiatric disease or did not wish to undergo CT imaging.
Fifty-four OSA patients were recruited who had been consecutively referred to a single surgeon as CPAP failure for salvage airway surgery opinion. No patients in this cohort had prior non-nasal airway surgery, with the exception of simple palatine tonsillectomy. All patients were advised to re-trial nasal or full-face CPAP (or both) for 3 months prior to consideration of surgical treatment (16). Following this, CPAP adherence was re-assessed in a multidisciplinary setting with an otolaryngologist and sleep physician. Patients who were unable to utilise CPAP in a meaningful manner as defined by less than 4 hours of recorded usage per night (or outright refusal) were considered persistently non-adherent to CPAP (2). As part of routine assessment prior to consideration of upper airway surgery, all 54 patients underwent CT neck with airway reconstruction protocol, detailed further below.
For our secondary hypothesis and in accordance with our power analysis, 23 consenting patients who had undergone CT neck imaging for reasons other than OSA were screened for sleep disordered breathing with the Berlin questionnaire (group C) (17). These patients were only considered if there were no categories where the score was positive and there was no history of snoring from the patient or their partner, indicating at most, low risk of OSA and likely minimal to no risk. The overall analysis included a total of 77 patient datasets for analysis (41, 13, and 23 respectively, see Figure 1).
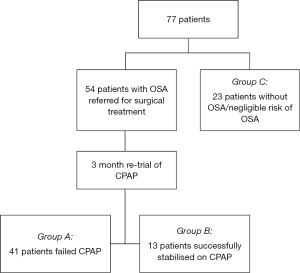
Patient demographics and metrics
Patient demographics were recorded including age, gender and body mass index (BMI). A complete sleep history was taken including Epworth Sleepiness Scale (ESS) (18) and Snoring Severity Scale (SSS) (19) questionnaires. Oral examination was performed to evaluate Friedman tongue grade, size of palatine tonsil and evidence of malocclusion. Awake nasendoscopy was utilised to assess upper airways collapse through dynamic manoeuvres (including Modified Mueller Manoeuvre and Woodson’s Hypotonic Method) and to evaluate presence of nasal obstruction, palatal phenotype (20), presence of lingual tonsil and position of the epiglottis. Repeat polysomnography was performed if the most recent study was over 24 months old. Key PSG measurements analysed included apnoea-hypopnoea index (AHI) and oxygen saturation nadir (Lsat). As the respiratory disturbance index (RDI) was not routinely reported in all PSG studies, this was not included in our analysis.
Airway reconstruction protocol CT scans were performed to measure tongue (lingual) SA, in inspiratory and expiratory phases, as a surrogate measure of tongue size. The lingual SA was assessed radiologically by measuring the midline cross-sectional area of the tongue in all three groups (according to Flinders Medical Center, Department of Medical Imaging 2010, Sleep Apnoea, A Radiographer’s Guide, see Supplementary file). Other anatomical markers measured included mandibular plane-hyoid distance (MPH), and sella-nasion distance to the maxillary alveolus (SNA) and anterior mandible (SNB).
Data analysis
Patient information was recorded using Microsoft Excel (Microsoft Corporation, WA, USA) and statistical analysis was undertaken using SPSS Statistics (IBM, SPSS Inc., Chicago, IL, USA) and Prism 8 (Graphpad Software Inc., La Jolla, CA, USA). Patients were analysed according to whether they failed CPAP (n=41, group A), were stabilised on CPAP (n=13, group B) or had negligible risk of OSA (n=23, group C). An alpha level of 0.05 was used for all statistical tests, with means, standard deviation and 95% confidence intervals (CI) tabulated. Variation and distribution within groups was assessed using Shapiro-Wilk test and Levene’s homogeneity of variances. Differences between groups according to age, BMI, reasons for CPAP failure, MPH, SNA, SNB and lingual SA were evaluated using one-way analysis of variance (ANOVA). Subgroup analysis between groups A, B, and C was conducted with Tukey post-hoc analysis and effect sizes calculated to obtain Hedges g value. Differences between group A and B according to gender and history of tonsillectomy were evaluated using Pearson Chi Squared test. Where available, CPAP pressures, AHI, ESS and SSS scores between groups was compared with two-tailed Independent Samples t-tests. Further correlation was also made between Friedman tongue grade and lingual SA using Spearman rank-order correlation.
Results
Of the 54 patients re-trialled on CPAP, 41 patients were unable to adhere to CPAP therapy (group A) whilst 13 were successfully stabilised on CPAP by the 12-week review (group B) (Figure 1).
Difference in terms of age, gender and BMI (Table 1)
Table 1
| Variable | Group A (failed CPAP) | Group B (stable on CPAP) | Group C (no OSA/negligible risk of OSA) |
|---|---|---|---|
| Age (n) | 41 | 13 | 22 |
| Mean ± std. deviation (years) | 46±11 | 53±15 | 51±18 |
| Range (years) | 22–67 | 28–76 | 21–88 |
| Gender, n (%) | 41 | 13 | 23 |
| Male | 33 (80.5%) | 8 (61.5%) | 11 (47.8%) |
| Female | 8 (19.5%) | 5 (38.5%) | 12 (52.2%) |
| BMI (n) | 40 | 13 | 18 |
| Mean ± std. deviation (kg/m2) | 29.3±3.1 | 26.1±4.1 | 25.8±4.1 |
| 95% CI (kg/m2) | 28.3–30.3 | 23.6–28.6 | 23.8–27.9 |
| Inspiratory lingual SA (n) | 41 | 13 | 23 |
| Mean ± std. deviation (mm2) | 3,446±482 | 3,154±447 | 2,998±435 |
| 95% CI (mm2) | 3,294–3,598 | 2,883–3,424 | 2,810–3,186 |
| Inspiratory MPH (n) | 41 | 13 | 23 |
| Mean ± std. deviation (mm) | 22.8±8.1 | 20.7±6.8 | 17.8±9 |
| 95% CI (mm) | 20.2–25.3 | 16.6–24.9 | 13.9–21.7 |
| SNA (n) | 41 | 13 | 23 |
| Mean ± std. deviation (mm) | 81.5±4.0 | 80.3±4.5 | 81.8±5.2 |
| 95% CI (mm) | 80.2–82.7 | 77.6–83.1 | 79.6–84.0 |
| SNB (n) | 41 | 13 | 23 |
| Mean ± std. deviation (mm) | 81.2±4.5 | 79.7±4.3 | 81.3±5.6 |
| 95% CI (mm) | 79.8–82.7 | 77.1–82.3 | 78.9–83.7 |
| Tonsillectomy history, n (%) | 41 | 13 | N/A |
| No tonsillar tissue | 14 (34.1%) | 10 (76.9%) | N/A |
| Tonsillar tissue present | 27 (65.9%) | 3 (23.1%) | N/A |
OSA, obstructive sleep apnoea; CPAP, continuous positive airway pressure; BMI, body mass index; MPH, mandibular-plane hyoid distance; SNA, sella-nasion distance to the maxillary alveolus; SNB, sella-nasion distance to the anterior mandible.
Ages were compared between groups. There were no outliers, as assessed by boxplot, and ages were normally distributed as assessed by Shapiro-Wilk test (P>0.05). No significant difference was found in terms of CPAP pressures and age using one-way ANOVA [F(2, 75) =1.533 P=0.223].
There was a significantly greater proportion of males (80.5%, 33/41) in the failed CPAP group with Pearson Chi squared test compared to the stable on CPAP group χ2 (2, n=77) =7.43, P=0.024. There was a statistical difference in average BMI between males and females (28.6 vs. 26.2, P=0.01).
There was significant difference in the BMI of patients across all groups [F(2, 68) =7.665, P=0.001] (Table 1). Those who failed CPAP were of significantly greater BMI than patients without OSA (group A vs. C, P=0.003, Tukey post-hoc analysis) and those stable on CPAP (group A vs. B, P=0.018, Tukey post-hoc analysis). BMI of patients without OSA was not significantly different compared to those stable on CPAP (group B vs. C, P=0.98).
Difference in lingual SA (Table 1 and Figure 2)
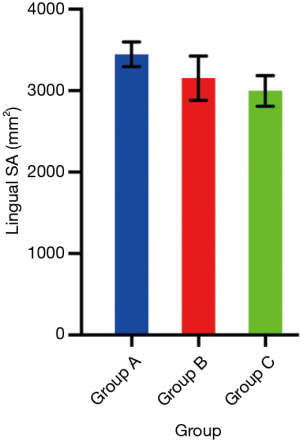
One-way analysis of variance (ANOVA) was conducted to determine if lingual SA on inspiration was different for the three groups: Failed CPAP (n=41, group A), Stable on CPAP (n=13, group B), patients without OSA (n=23, group C). Inspiration was chosen to ensure congruency in phase of respiration across the patient cohort. There were no outliers, as assessed by boxplot; data was normally distributed for each group, as assessed by Shapiro-Wilk test (P>0.05); and there was homogeneity of variances, as assessed by Levene’s test (P=0.59). Data is presented as mean ± standard deviation.
Lingual SA on inspiration was statistically significantly different between groups [F(2, 74) =7.35, P=0.001]. Lingual SA on inspiration was greater in those who failed CPAP, than those stable on CPAP, and patients without OSA (Table 1). Tukey post hoc analysis revealed that the difference between those who failed CPAP and patients without OSA was statistically significant (group A vs. C, P=0.001), but no other group differences were statistically significant (group A vs. B, P=0.12, group B vs. C, P=0.60).
Effect size was calculated between patients who failed CPAP and those who stabilised on CPAP. Hedge’s g value was 0.62, a greater than moderate effect (or difference between groups). Effect size between patients who failed CPAP and those without OSA was g=0.96, a large effect (or difference between groups).
One-way ANOVA was also conducted on MPH distance on inspiration [F(2, 74) =2.7, P=0.074], SNA [F(2, 74) =0.49, P=0.61] and SNB [F(2, 74) =0.59, P=0.56] distances but none of these group differences were statistically significant (Table 1).
Difference in terms of AHI, Lsat, ESS and SSS scores
There were no significant differences between the failed CPAP and stable on CPAP groups identified on Independent Samples t-test in terms of AHI [t(51) =0.73, P=0.47], Lsat [t(53) =0.48, P=0.63], ESS [t(49) =0.52, P=0.60] or SSS [t(43) =0.372, P=0.71].
Difference in terms of anatomical features on clinical examination
Participants who were stabilised on CPAP were significantly more likely to have had previous tonsillectomy with Friedman palatine tonsil size 0 on examination (76.9%, 10/13), as measured with Pearson Chi squared test compared to the failed CPAP group (34.1%, 14/41), χ2(1, n=54) =7.32, P=0.0068 (Table 1).
Friedman tongue grade was not predictive of lingual SA with Spearman rank-order correlation (rs=0.04, P=0.78). Adherence to CPAP was not associated with greater Friedman tongue grade as measured by Independent Samples t-test with Welch’s correction [t(1) =0.48, P=0.64].
Difference in terms of CPAP pressures
CPAP pressure data was not available for all patients, either due to outright patient refusal to trial CPAP or a lack of data at the time of enrolment. Where data was available, pressures were not significantly greater in participants who failed CPAP (n=14) compared with those who stabilised on CPAP (n=8) using Independent Samples t-test with Welch’s correction [t(20) =1.17, P=0.26].
Discussion
Key findings
CT scanning is a widely available and relatively inexpensive modality compared with MRI that can be used in assessing tongue SA and airway anatomy in patients with OSA.
There was no significant difference between patients who failed CPAP (3,446 mm2) compared with those who stabilised on CPAP (3,154 mm2), although the mean SA was greater and the effect size was more than moderately different. This is likely to be clinically relevant and, as others have noted, important for future research (21) in evaluating whether measures to reduce tongue size might lead to greater CPAP adherence. Our results demonstrate that patients who fail CPAP have significantly greater mean lingual SA (3,446 mm2) when compared to patients without OSA (2,998 mm2). Previously, >2,800 mm2 has been considered a cut-off for defining macroglossia (22).
We found a correlation between lingual SA in patients with OSA and increased BMI. Those who failed CPAP had a significantly higher mean BMI (28.9 kg/m2) than those stabilized on CPAP (26.1 kg/m2) and also those without OSA group (25.8 kg/m2). Male patients were more likely to fail CPAP, have greater lingual SA and greater BMI.
Variation in lingual SA between inspiratory and expiratory phases of breathing was not shown to have any correlation with adherence to CPAP nor with having OSA.
Clinical assessment of Friedman Tongue Grade was not a reliable method of correlating tongue SA or determining CPAP failure. However, the presence of tonsillar tissue was associated with an increased likelihood of failing CPAP adherence. Although alluded to in the literature (23,24), such a finding is unique and may have significant clinical importance in considering tonsillectomy as a pathway to improve CPAP adherence.
Limitations of the study
There is an inherent population bias by virtue of being recruited from a sleep surgery referral center. Hence, a high proportion of patients are likely to be “surgically inclined” and may be more likely to fail or outright refuse therapy with CPAP compared with the general population diagnosed with OSA. Nevertheless, there are no non-clinically biased community cohort studies that explore macroglossia as a factor in CPAP non-adherence, making this study the solitary example for future evaluations.
Recruitment of patients with no OSA was performed without polysomnography. This bias was minimised with the use of the Berlin questionnaire, a validated screening tool for OSA with moderate to high sensitivity and utility as a “rule out” test (17,25). Patients were only considered suitable for the comparator group if their Berlin questionnaire score was negative in each category and did not report a history of snoring either themselves or from their partner. Not all data was available for control patients (such as inspiratory versus expiratory volumes) and despite satisfying the minimum cohort size based on the pre-study power analysis, groups were unequal and these factors limited the power of our analysis of corollary data.
Comparison with literature
Patients with CPAP side effects are more likely not to adhere to long term use. Engleman et al. initially described the commonly reported symptoms of nasal congestion, upper airway dryness, claustrophobia and consequences of excessive pressures from the system (26). Chai-Coetzer et al. showed usage habits of CPAP at 1 month can be predictive of adherence at 12 months (27). Recently, Nadal et al. listed factors for non-adherence in the literature including higher ESS scores, BMI and CPAP pressures (28), with a comparable finding demonstrated in our BMI results. Our results did not demonstrate a link between CPAP pressures and adherence, although available data was limited.
CPAP pressures are closely linked to upper airway anatomy. Lai et al. demonstrated anatomical predictors including the updated Friedman tongue position and hyoid-mental distance were correlated with determining optimal CPAP pressure (29). Park et al. found associations between failure of CPAP and septal deviation, inferior turbinate hypertrophy, tonsillar hypertrophy and long palatal phenotypes (5). These are hypothesised to reduce nasal airflow, increase upper airway resistance, requiring higher CPAP pressures and thus predispose patients to non-adherence. Our results demonstrate a corresponding higher CPAP stabilisation rate in patients with previous tonsillectomy, compared to those who still had palatine tonsillar tissue.
Tongue size is another component of upper airway anatomy that has been studied. Elevated BMI is often a confounding factor in studies evaluating OSA, with known associations between adherence to CPAP and BMI (5). As an example and in contrast to our results, Wild et al. demonstrated higher adherence to CPAP with higher BMI (30). Ahn et al. showed a correlation between the severity of OSA, oxygen saturation nadir and BMI to tongue volume both assessed clinically and with volumetric CT analysis (13). Kim et al. utilised MRI to evaluate tongue fat in obese patients with OSA compared to obese patients without, and found significantly increased tongue fat content in patients with OSA, but importantly highlighted that tongue SA or volume was not directly correlated with elevated BMI (9). Our results identify a link between surrogate measures of tongue size (tongue SA) and non-adherence to CPAP, but more data is required to contrast patients who remain adherent to CPAP to those who do not.
CT has previously been employed to assess cross-sectional area of the airway lumen (15) as well as soft tissue and bony measurements (13). CT is especially useful for defining cephalometric bony fixed point measures for surgical planning and is easily tolerated by patients due to shorter imaging sequences, but is disadvantaged by the radiation dosage and the poorer resolution of soft tissue compared to MRI (31). However, it remains an inexpensive, efficient and accessible modality for clinicians to evaluate patients who are considering upper airway intervention for their OSA.
Conclusions
Our results suggest a difference in inspiratory lingual SA may have clinical significance in adherence to CPAP. Our findings might offer potential targets for high level trials in tongue reduction surgery, such as radiofrequency tongue channelling, midline glossectomy and submucosal lingualplasty. Additionally, tonsillectomy could be considered for further prospective research in increasing the likelihood of adherence to CPAP.
Adult patients with OSA who fail to adhere to CPAP under appropriate supervision, can be considered for assessment of increased tongue SA as a contributory factor. Previous tonsillectomy, lower BMI and female gender are associated with a greater likelihood of adherence to CPAP. Our findings provide a template for subsequent research into both identifying contributors to CPAP failure and potential interventions to improve CPAP adherence.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ajo-20-23
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo-20-23). SGM serves as an unpaid editorial board member of Australian Journal of Otolaryngology from Jan 2019 to Dec 2020. SGM reports Proctor for Genio-Nyxoah Implantation (broadly a “tongue therapy” in OSA), NH&MRC CI on Airway Surgery RCT that included a tongue intervention, GPRWF Conjoint Grant investigating airway treatments that might theoretically involve tongue therapy. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Wollongong/Illawarra Shoalhaven local Health District Health and Medical Human Research Ethics Committee (HE11/468), and was conducted in accordance with the NHMRC National Statement on Ethical Conduct in Human Research. The authors confirm that written informed consent was obtained from patients for publication of this article.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med 2019;15:301-34. [Crossref] [PubMed]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008;5:173-8. [Crossref] [PubMed]
- Love RL, Naughton MT, Cistulli P, et al. Complications and safe prescription of interventions for adult sleep disordered breathing in Australia. Aust J Otolaryngol 2019;2:9. [Crossref]
- Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath 2010;14:323-35. [Crossref] [PubMed]
- Park P, Kim J, Song YJ, et al. Influencing factors on CPAP adherence and anatomic characteristics of upper airway in OSA subjects. Medicine (Baltimore) 2017;96:e8818. [Crossref] [PubMed]
- Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol 2011;268:1233-6. [Crossref] [PubMed]
- Lee RW, Sutherland K, Chan AS, et al. Relationship between surface facial dimensions and upper airway structures in obstructive sleep apnea. Sleep 2010;33:1249-54. [Crossref] [PubMed]
- Do KL, Ferreyra H, Healy JF, et al. Does tongue size differ between patients with and without sleep-disordered breathing? Laryngoscope 2000;110:1552-5. [Crossref] [PubMed]
- Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep 2014;37:1639-48. [Crossref] [PubMed]
- Genta PR, Schorr F, Eckert DJ, et al. Upper airway collapsibility is associated with obesity and hyoid position. Sleep 2014;37:1673-8. [Crossref] [PubMed]
- Li S, Qin Y, Wu D. Lingual-occlusal surface position predicts retroglossal obstruction in patients with obstructive sleep apnea hypopnea syndrome. Acta Otolaryngol 2015;135:1146-51. [Crossref] [PubMed]
- Barrera JE, Pau CY, Forest VI, et al. Anatomic measures of upper airway structures in obstructive sleep apnea. World J Otorhinolaryngol Head Neck Surg 2017;3:85-91. [Crossref] [PubMed]
- Ahn SH, Kim J, Min HJ, et al. Tongue volume influences lowest oxygen saturation but not apnea-hypopnea index in obstructive sleep apnea. PLoS One 2015;10:e0135796. [Crossref] [PubMed]
- Abramson Z, Susarla S, August M, et al. Three-dimensional computed tomographic analysis of airway anatomy in patients with obstructive sleep apnea. J Oral Maxillofac Surg 2010;68:354-62. [Crossref] [PubMed]
- Barkdull GC, Kohl CA, Patel M, et al. Computed tomography imaging of patients with obstructive sleep apnea. Laryngoscope 2008;118:1486-92. [Crossref] [PubMed]
- MacKay SG, Weaver EM. Surgery for adult obstructive sleep apnoea. Med J Aust 2013;199:450-1. [Crossref] [PubMed]
- Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485-91. [Crossref] [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [Crossref] [PubMed]
- Lim PV, Curry AR. A new method for evaluating and reporting the severity of snoring. J Laryngol Otol 1999;113:336-40. [Crossref] [PubMed]
- Woodson BT. A method to describe the pharyngeal airway. Laryngoscope 2015;125:1233-8. [Crossref] [PubMed]
- Karadaghy OA, Hong H, Scott-Wittenborn N, et al. Reporting of effect size and confidence intervals in JAMA Otolaryngology–Head & Neck Surgery. JAMA Otolaryngol Head Neck Surg 2017;143:1075-80. [Crossref] [PubMed]
- Chabolle F, Wagner I, Blumen MB, et al. Tongue base reduction with hyoepiglottoplasty: a treatment for severe obstructive sleep apnea. Laryngoscope 1999;109:1273-80. [Crossref] [PubMed]
- Martinho FL, Zonato AI, Bittencourt LRA, et al. Obese obstructive sleep apnea patients with tonsil hypertrophy submitted to tonsillectomy. Braz J Med Biol Res 2006;39:1137-42. [Crossref] [PubMed]
- Nakata S, Miyazaki S, Ohki M, et al. Reduced nasal resistance after simple tonsillectomy in patients with obstructive sleep apnea. Am J Rhinol 2007;21:192-5. [Crossref] [PubMed]
- Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology 2008;108:822-30. [Crossref] [PubMed]
- Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax 1994;49:263-6. [Crossref] [PubMed]
- Chai-Coetzer CL, Luo YM, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep 2013;36:1929-37. [Crossref] [PubMed]
- Nadal N, de Batlle J, Barbé F, et al. Predictors of CPAP compliance in different clinical settings: primary care versus sleep unit. Sleep Breath 2018;22:157-63. [Crossref] [PubMed]
- Lai CC, Friedman M, Lin HC, et al. Clinical predictors of effective continuous positive airway pressure in patients with obstructive sleep apnea/hypopnea syndrome. Laryngoscope 2015;125:1983-7. [Crossref] [PubMed]
- Wild MR, Engleman HM, Douglas NJ, et al. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J 2004;24:461-5. [Crossref] [PubMed]
- Tipnis SV, Spampinato MV, Hungerford J, et al. Thyroid doses and risks to adult patients undergoing neck CT examinations. AJR Am J Roentgenol 2015;204:1064-8. [Crossref] [PubMed]
Cite this article as: Lam ME, Kitipornchai L, Chan L, Creber NJ, Hayward NJ, Jones AC, Petersen AJ, Sarkissian L, MacKay SG. Assessment of macroglossia as a cause of failed continuous positive airway pressure adherence. Aust J Otolaryngol 2020;3:25.


