Cost effectiveness of posterior epistaxis management using a gelatin-thrombin matrix or Rapid Rhino
Introduction
Epistaxis can be classified as either anterior (90% of cases) or posterior (10%) based on the location of bleeding with the division between anterior and posterior nasal septum lying at the pyriform aperture (1,2). Anterior haemorrhages can often be managed with basic first aid and chemical- or electro-cautery (1,3).
In situations where these methods are unsuccessful or not practical, such as cases of posterior epistaxis, nasal packing may be required (1,4). Traditionally this involved the use of lubricated ribbon gauze or the use of a Foley catheter which is inserted along the nasal passage floor to the posterior nasopharynx with Kaltostat or ribbon gauze (1,5). However modern packs such as the Rapid Rhino provide easier and more effective alternatives and have become the mainstay treatment for posterior epistaxis in many centres (6). The duration of non-absorbable nasal packing is not specified in the literature, though in practice times range between 24–72 hours with oral antibiotic prophylaxis to cover against toxic shock syndrome (6,7).
Whilst representing an important management option for posterior epistaxis, packing with the Rapid Rhino has several drawbacks. These include requiring hospital admission whilst the packs are in-situ, trauma on insertion, patient discomfort, tissue necrosis, toxic shock syndrome and failure (1,4). Absorbable packing agents such as Nasopore®, Surgicel® or haemostatic glue have not been well researched for the primary treatment of posterior epistaxis, with most research focusing on utilization in the post-operative setting (7). Both arterial embolization and ligation have high success rates, though require significant resources, expertise and equipment (8-11).
FloSeal (Baxter Corporation, Deerfield, IL, USA) is a biological dissolvable haemostatic gelatin-thrombin matrix that is frequently used in endoscopic sinus surgery and epistaxis. It has been shown to be efficacious for posterior epistaxis and general haemorrhage control in other surgical procedures (11-19). This gelatin-thrombin matrix is easy to apply, dissolvable and a proven haemostatic agent and thus has the potential to treat posterior epistaxis without the need for hospital admission (11). Furthermore, it has been shown to be a successful treatment for posterior epistaxis with lower morbidity compared with other methods of haemostatic control (11). Whilst an effective treatment modality, there is little evidence investigating its cost-effectiveness in the Australian healthcare setting. Thus, this study aimed to assess the cost-effectiveness of a gelatin-thrombin matrix in the treatment of posterior epistaxis when compared with a dual balloon Rapid Rhino.
Methods
A decision tree model was constructed to evaluate the cost-effectiveness of the management of posterior epistaxis using a gelatin-thrombin matrix (FloSeal) compared to the dual balloon Rapid Rhino (Figure 1). The decision tree model assumed patients managed with a Rapid Rhino were admitted to hospital and that those managed successfully with a gelatin-thrombin matrix were discharged from the emergency department. In cases where gelatin-thrombin matrix failed, it was assumed patients would be admitted for operative management (Figure 1). Estimates for the average cost of a ward bed admission, initial Emergency Department assessment and theatre were based on the financial year 2015/16 annual Victorian Cost Data Collection (VCDC) Cost Submission which included 130 patients with epistaxis discharged from Box Hill Hospital. Costs related to Rapid Rhino and the gelatin-thrombin matrix (FloSeal) were sourced from the product manufacturer. The cost of cephalexin was based on estimates from the pharmaceutical benefits scheme (PBS) (20).
Effectiveness for each modality was calculated using previously published data. A literature search was undertaken to identify studies that reported effectiveness rates for either FloSeal or the Rapid Rhino in posterior epistaxis. Random effects meta-analysis was used to calculate a cross-study pooled estimates to account for sampling error and heterogeneity in prevalence and incidence estimates (21).
This study was approved by the Eastern Health Ethics Committee (reference LR88/2016).
Statistical analysis
One-way sensitivity analyses (varying costs by ±25%) and a probabilistic sensitivity analysis was performed using second-order Monte Carlo simulation, a method to account for joint uncertainties in the parameter estimates (22). This involved randomly selected values from each input parameter’s distribution and generated results for that combination of values. This process was repeated 10,000 times. Gamma distributions were used for costs, and beta distributions for the transition probabilities. Tornado diagrams (which visually show the impact of varying the values of one parameter at a time) were produced to summarize the one-way sensitivity analyses and probabilistic sensitivity analyses results were summarized using acceptability curves. Acceptability curves summarizes the probability of an intervention to be more cost-effective than the comparator, according to the willingness-to-pay threshold (how much a funder is willing to pay to obtain the health outcome). The incremental cost-effectiveness ratio (ICER) was calculated using the following formula:
Results
Literature review
There was limited published data regarding the efficacy of nasal packing with the Rapid Rhino or gelatin-thrombin matrix for the treatment of posterior epistaxis (23,24). A systematic search using MEDLINE and EMBASE was conducted to identify studies that reported on efficacy rates of gelatin-thrombin matrix or the Rapid Rhino for the treatment of posterior epistaxis. The following search terms were used: (FloSeal OR Gelatin-thrombin matrix OR Rapid Rhino) AND Epistaxis. Only papers published since January 1st 2000 were included. A total of 101 papers from EMBASE and 34 papers from MEDLINE were independently screened. Case reports and case series were excluded. Of these, five studies were found that reported efficacy rates for either gelatin-thrombin matrix or Rapid Rhino for the treatment of posterior epistaxis (5,11,12,25,26). Our efficacy data for posterior epistaxis was based on three studies for the Rapid Rhino and two for gelatin-thrombin matrix (5,11,12,25,26) (Table 1). One other study was also identified that investigated the efficacy of a gelatin-thrombin matrix in posterior epistaxis with a success rate of 76% (27). However, it was not used in our model as it did not specifically differentiate between anterior and posterior epistaxis.
Table 1
| Costs (A$2,017) | Estimate | Sensitivity analyses | Reference |
|---|---|---|---|
| FloSeal | A$420 | Gamma distribution | Baxter Corporation, Deerfield, IL, USA |
| α =70.56 | |||
| λ =0.17 | |||
| Rapid Rhino | A$47 | Gamma distribution | Smith & Nephew, Austin, TX, USA |
| α =61.36 | |||
| λ =1.31 | |||
| Rapid Rhino antibiotic prophylaxis* | A$19 | Gamma distribution | PBS 3119E (20) |
| α =90.25 | |||
| λ =4.75 | |||
| Ward bed (per admission) | A$1,683 | Gamma distribution | VCDC cost submission |
| α =64.23 | |||
| λ =0.04 | |||
| Theatre | A$1,110 | Gamma distribution | VCDC cost submission |
| α =64.00 | |||
| λ =0.06 | |||
| Probability of haemostasis | |||
| Gelatin-thrombin matrix | 80% | Beta distribution | (11,12) |
| α =12.00 | |||
| β =3.00 | |||
| Rapid Rhino | 65% | Beta distribution | (5,25,26) |
| α =14.14 | |||
| β =7.61 |
*, cephalexin 500 mg four times daily for 5 days (PBS 3119E) (20).
Effectiveness of haemostasis
Pooled estimates of effectiveness were calculated using estimates from previously published studies investigating either the Rapid Rhino or the gelatin-thrombin matrix to treat posterior epistaxis. The gelatin-thrombin matrix [80%, 95% confidence intervals (CI): 63–93] demonstrated greater effectiveness when compared with the Rapid Rhino (65%, 95% CI: 57–72) when used to treat posterior epistaxis (5,11,12,25,26) (Table 1).
Cost
The mean cost of the gelatin-thrombin matrix was A$953 per patient (standard deviation A$226) and the mean cost for the Rapid Rhino was A$1,004 (standard deviation A$138). This was based on cost of the product itself, hospital admission and adjunct medications (i.e., oral antibiotics). The gelatin-thrombin matrix demonstrated more favorable results than Rapid Rhino in terms of cost-savings and haemostasis effectiveness.
Cost effectiveness
These results equated to a cost saving of A$292 per posterior epistaxis case managed if using the gelatin-thrombin matrix instead of Rapid Rhino. The tornado diagram identifies which factors are most influential in changing the cost per case of posterior epistaxis managed (Figure 2). This diagram demonstrates that the most influential factors are the probability of haemostasis to Rapid Rhino, followed by the costs of the inpatient stay and cost of the gelatin-thrombin matrix (Figure 2).
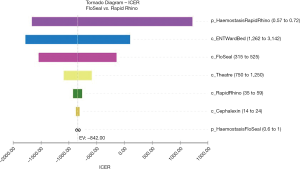
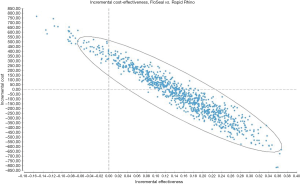
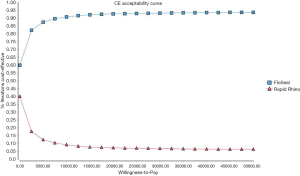
Figure 3 is a scatterplot on the cost-effectiveness plane of 10,000 ICERs using probabilistic sensitivity analyses. It demonstrates that a substantial number of ICERs are in the cost-saving (i.e., South-East) quadrant. This was reinforced by the acceptability curve (Figure 4) showing that the gelatin-thrombin matrix had a higher probability of being cost-effective compared to Rapid Rhino.
Discussion
Key findings
This study investigated and compared the cost-effectiveness of the dual balloon Rapid Rhino nasal pack and a gelatin-thrombin matrix haemostatic agent in the treatment of posterior epistaxis. The average cost of treatment using the Rapid Rhino was A$1,004 compared to the gelatin-thrombin matrix which cost, on average, A$961. Our model suggests that the gelatin-thrombin matrix is likely to be cost-saving compared to Rapid Rhino for the control of posterior haemostasis from an Australian healthcare provider perspective. The main reason for this appears to be reduced hospital bed-days, and improved efficacy.
Strengths and limitations
The gelatin-thrombin matrix is currently not a well utilized resource as a first-line management tool for posterior epistaxis. The Rapid Rhino system is far more commonly used in the management of posterior epistaxis in Australian healthcare centres. Whilst the efficacy for posterior epistaxis management using a gelatin-thrombin matrix or the Rapid Rhino has been investigated in previous studies (8,12,13,24,26), this is the first comprehensive analysis of cost-effectiveness of both products in the Australian healthcare setting.
Whilst this study provided a comprehensive cost-analysis of two important treatment modalities for managing posterior epistaxis, there were several limitations. The costing data was obtained based on patients treated within a single health network in Victoria, Australia. Specific costs of hospital stay, and operative management may vary between hospitals and regions. Similarly, treatment protocols are not standardised across healthcare centres in Australia which may compound cost variances across sites depending on local practices.
Heterogeneity amongst patients with posterior epistaxis and the way in which different treatments were administered within the literature also represented another limitation. For example, Kilty et al. used a Foley catheter along with a gelatin-thrombin matrix to control epistaxis and in cases of initial failure would administer a second 5 mL syringe (11). This method of gelatin-thrombin matrix administration differed when compared to Cote et al., where nasendoscopy was used to identify a posterior bleeding point and apply the gelatin-thrombin matrix directly to the area (12). For the purposes of this cost study it was assumed only one 5 mL syringe of FloSeal would be used without the use of adjuncts such as a Foley catheter or nasendoscopy. Similarly, whether patients with non-dissolvable packs should be discharged home was another area of contention amongst published studies. Published guidelines suggest patients treated with haemostatic agents or dissolvable packs are safe to be discharged home (6). However, there is no clear consensus on whether patients managed with non-dissolvable packs are safe to be discharged, particularly in cases where posterior packing is required (28). Van Wyk et al. advocated that whilst it is reasonable to discharge patients with anterior packs in-situ, posterior packing should be performed only by a specialist trained in the procedure and warrants admission (28). Our institution has taken a similar approach whereby all patients requiring posterior nasal packing were managed as inpatients, thus this protocol was used in the cost-benefit modelling for this study.
The data investigating effectiveness for the gelatin-thrombin matrix was not equivocally in favour of this system. Khan et al. also evaluated the use of a gelatin-thrombin matrix in posterior epistaxis in a series of 33 patients over a 2-month period. Only three cases demonstrated complete haemostasis without the need for further interventions and no readmission with epistaxis within 7 days after its application (29). Similarly, in a prospective clinical study which included a narrative literature review, Wakelam et al. contended that the literature largely supported the notion that posterior epistaxis can often be difficult to control, even with a gelatin-thrombin matrix, and that management with endoscopic sphenopalatine artery ligation is emerging as a more cost-effective and definitive measure of control (24). As the Khan reference was a case series it was not included in our final analysis. However, if we include it, the pooled effectiveness for the gelatin-thrombin matrix will be 54% (95% CI: 5–99%). This results in a mean cost of A$1,653 for the gelatin-thrombin matrix (standard deviation A$249), and A$1,002 for Rapid Rhino (standard deviation A$138). The administration of the gelatin-thrombin matrix is user dependent, and this may have been one explanation for the high rates of failure observed by Khan et al. (29).
Whilst both modalities appear to be at least comparable in terms of cost in the Australian setting there are several limitations to its use in the clinical setting. These include ease of access of gelatin-thrombin matrix (particularly on the ward and in the emergency department) and ease of use amongst emergency department physicians who may not have much experience with the product. Our experience with gelatin-thrombin matrix in the clinical setting is primarily limited to use intraoperatively, and even in this setting a learning curve exists in relation to competent and proper use of the product. Future research could thus focus on the practicalities of introducing gelatin-thrombin matrix as a treatment modality in the emergency department setting in Australian centres as well as prospective head-to-head trials to assess both products once implemented.
Conclusions
The gelatin-thrombin matrix provides a viable treatment alternative given its comparable efficacy profile when compared with the Rapid Rhino. A significant barrier to uptake of the gelatin-thrombin matrix in treating posterior epistaxis has been cost of the product itself. However, when its reduced requirement for hospital admission is considered, it becomes a more attractive option. Thus, gelatin-thrombin matrix should be considered in the first-line management of posterior epistaxis along with other traditional nasal packing techniques.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2020.03.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Eastern Health Ethics Committee (reference LR88/2016).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yau S. An update on epistaxis. Aust Fam Physician 2015;44:653-6. [PubMed]
- Gifford TO, Orlandi RR. Epistaxis. Otolaryngol Clin North Am 2008;41:525-36. viii. [Crossref] [PubMed]
- Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J 2005;81:309-14. [Crossref] [PubMed]
- Kucik CJ, Clenney T. Management of epistaxis. Am Fam Physician 2005;71:305-11. [PubMed]
- Soyka MB, Nikolaou G, Rufibach K, et al. On the effectiveness of treatment options in epistaxis: an analysis of 678 interventions. Rhinology 2011;49:474-8. [PubMed]
- Integrate, National ENTTRN. The British Rhinological Society multidisciplinary consensus recommendations on the hospital management of epistaxis. J Laryngol Otol 2017;131:1142-56. [Crossref] [PubMed]
- Bequignon E, Verillaud B, Robard L, et al. Guidelines of the French Society of Otorhinolaryngology (SFORL). First-line treatment of epistaxis in adults. Eur Ann Otorhinolaryngol Head Neck Dis 2017;134:185-9. [Crossref] [PubMed]
- Bent JP 3rd, Wood BP. Complications resulting from treatment of severe posterior epistaxis. J Laryngol Otol 1999;113:252-4. [Crossref] [PubMed]
- Walen SG, Rudmik LR, Lipkewitch S, et al. Training, practice, and referral patterns in rhinologic surgery: survey of otolaryngologists. J Otolaryngol Head Neck Surg 2010;39:297-303. [PubMed]
- Elden L, Montanera W, Terbrugge K, et al. Angiographic embolization for the treatment of epistaxis: a review of 108 cases. Otolaryngol Head Neck Surg 1994;111:44-50. [Crossref] [PubMed]
- Kilty SJ, Al-Hajry M, Al-Mutairi D, et al. Prospective clinical trial of gelatin-thrombin matrix as first line treatment of posterior epistaxis. Laryngoscope 2014;124:38-42. [Crossref] [PubMed]
- Côté D, Barber B, Diamond C, et al. FloSeal hemostatic matrix in persistent epistaxis: prospective clinical trial. J Otolaryngol Head Neck Surg 2010;39:304-8. [PubMed]
- Bak JB, Singh A, Shekarriz B. Use of gelatin matrix thrombin tissue sealant as an effective hemostatic agent during laparoscopic partial nephrectomy. J Urol 2004;171:780-2. [Crossref] [PubMed]
- Cillo JE Jr, Sinn D, Truelson JM. Management of middle meningeal and superficial temporal artery hemorrhage from total temporomandibular joint replacement surgery with a gelatin-based hemostatic agent. J Craniofac Surg 2005;16:309-12. [Crossref] [PubMed]
- Durrani OM, Fernando AI, Reuser TQ. Use of a novel topical hemostatic sealant in lacrimal surgery: a prospective, comparative study. Ophthal Plast Reconstr Surg 2007;23:25-7. [Crossref] [PubMed]
- Ellegala DB, Maartens NF, Laws ER Jr. Use of FloSeal hemostatic sealant in transsphenoidal pituitary surgery: technical note. Neurosurgery 2002;51:513-5; discussion 5-6. [Crossref] [PubMed]
- Weaver FA, Hood DB, Zatina M, et al. Gelatin-thrombin-based hemostatic sealant for intraoperative bleeding in vascular surgery. Ann Vasc Surg 2002;16:286-93. [Crossref] [PubMed]
- Renkens KL Jr, Payner TD, Leipzig TJ, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine (Phila Pa 1976) 2001;26:1645-50. [Crossref] [PubMed]
- Jo SH, Mathiasen RA, Gurushanthaiah D. Prospective, randomized, controlled trial of a hemostatic sealant in children undergoing adenotonsillectomy. Otolaryngol Head Neck Surg 2007;137:454-8. [Crossref] [PubMed]
- Pharmaceutical Benefits Scheme. Available online: www.pbs.gov.au/pbs/home. Accessed 24 February 2018.
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [Crossref] [PubMed]
- Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--6. Value Health 2012;15:835-42. [Crossref] [PubMed]
- Iqbal IZ, Jones GH, Dawe N, et al. Intranasal packs and haemostatic agents for the management of adult epistaxis: systematic review. J Laryngol Otol 2017;131:1065-92. [Crossref] [PubMed]
- Wakelam OC, Dimitriadis PA, Stephens J. The use of FloSeal haemostatic sealant in the management of epistaxis: a prospective clinical study and literature review. Ann R Coll Surg Engl 2017;99:28-30. [Crossref] [PubMed]
- Moumoulidis I, Draper MR, Patel H, et al. A prospective randomised controlled trial comparing Merocel and Rapid Rhino nasal tampons in the treatment of epistaxis. Eur Arch Otorhinolaryngol 2006;263:719-22. [Crossref] [PubMed]
- Gudziol V, Mewes T, Mann WJ. Rapid Rhino: A new pneumatic nasal tamponade for posterior epistaxis. Otolaryngol Head Neck Surg 2005;132:152-5. [Crossref] [PubMed]
- Murray S, Mendez A, Hopkins A, et al. Management of Persistent Epistaxis Using Floseal Hemostatic Matrix vs. traditional nasal packing: a prospective randomized control trial. J Otolaryngol Head Neck Surg 2018;47:3. [Crossref] [PubMed]
- Van Wyk FC, Massey S, Worley G, et al. Do all epistaxis patients with a nasal pack need admission? A retrospective study of 116 patients managed in accident and emergency according to a peer reviewed protocol. J Laryngol Otol 2007;121:222-7. [Crossref] [PubMed]
- Khan MK, Reda El Badawey M, Powell J, et al. The utility of FloSeal haemostatic agent in the management of epistaxis. J Laryngol Otol 2015;129:353-7. [Crossref] [PubMed]
Cite this article as: Toppi J, Chan SW, Pratap U, Kumar S, Phan R, Magarey M, Ong JJ. Cost effectiveness of posterior epistaxis management using a gelatin-thrombin matrix or Rapid Rhino. Aust J Otolaryngol 2020;3:14.