
Management of occult neck disease in metastatic squamous cell carcinoma to the parotid gland
Introduction
In Australia, the most common malignancy of the parotid gland is metastatic cutaneous squamous cell carcinoma (cSCC), typically arising from cutaneous head and neck disease (1). It has been demonstrated that metastatic disease to the parotid and cervical neck confers quite a poor prognosis (2). The presence of metastatic cSCC to the parotid represents at least stage III disease, typically treated with bimodal therapy in the form of surgery and post-operative radiation therapy (3). In 2002, O’Brien presented a staging system for metastatic cSCC to the parotid, which separates parotid “P stage” from neck “N stage” (1). Despite not forming part of the American Joint Committee on Cancer staging system, this delineation continues to influence current treatment strategies.
At present, there is no consensus on the optimal management of potential occult neck metastases in the parotid positive and neck negative (P+N0) patient (4,5). Australian series have reported occult neck metastases rates between 15 and 35% (6-8). The presence of preoperatively known positive neck disease (P+N+) typically mandates parotidectomy and surgical clearance of the nodal basin via formal neck dissection. However, in the P+N0 patient with no clinical or radiological evidence of neck disease, given the parotid is managed surgically, the neck may be treated surgically, treated with radiation, or a combination of both.
This retrospective case series will review the management of P+N0 patients. The principle aim of the study was to determine whether elective neck dissection conferred disease-free or overall survival benefits in this series of patients.
Methods
Institutional Human Research Ethics Committee approval was obtained (approval number RPH HREC 15-088). This study is a retrospective case series of patients with metastatic cutaneous SCC to the parotid gland without preoperative evidence of neck nodal metastases. Prospectively maintained theatre management software was interrogated to identify all patients who underwent “parotidectomy” during the study period. Histopathology reports were then reviewed to identify patients with SCC. Patients were eligible for inclusion if they underwent parotid surgery for metastatic squamous cell carcinoma at one of Western Australia’s three tertiary hospitals, between June 2002 and June 2013. All cases were treated with curative intent. Patients for whom there was insufficient data on chart review were excluded (n=13).
Demographic data was cross-referenced using name, sex, date of birth, and unique medical record number. Clinical information was coded for age (years), sex (male:female), immune competence (intact:compromised), duration of presenting complaint (weeks), presence of active primary lesion (present:absent), P-stage (O’Brien—P1:P2+), and facial nerve involvement (intact:compromised) (see Table 1). Radiologic imaging and reporting systems were reviewed to confirm parotid staging and the absence of cervical metastases, in conjunction with clinical examination findings at the time of presentation. Surgical and peri-operative data was also coded (see Table 1). Surgical notes were coded for wait time for surgery (days), extent of parotidectomy (superficial:total), levels of neck dissection (if completed), and duration of operation. Histologic reports were coded for margin (mm). Inpatient notes were coded for length of stay (days) and return to theatre (yes:no). Post-operative radiation was coded (present:absent).
Table 1
| Variables | Neck dissection (n=62) | No neck dissection (n=33) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 71.8±11.3 | 72.8±12.7 | 0.690 |
| Gender (males, %) | 81 | 76 | 1.000 |
| Immunocompromise (%) | 40 | 27 | 0.260 |
| Active primary (%) | 21 | 3 | 0.030 |
| P stage (2+, advanced) (%) | 60 | 33 | 0.018 |
| Duration presenting complaint (weeks), median [IQR] | 8 [4–12] (n=51) | 8 [5–12] (n=28) | 0.540 |
| Facial nerve paresis (grade 2–6) | 10% (n=48) | 14% (n=29) | 0.720 |
| Parotidectomy (superficial) | 60% | 79% | 0.070 |
| Post-operative radiotherapy | 76% | 85% | 0.430 |
| Margins involved (<1 mm) | 66% | 52% | 0.190 |
| Wait time for surgery (days), median [IQR] | 26 [17–36] | 23 [15–35] | 0.420 |
| Duration of operation (min), median [IQR] | 270 [210–340] | 169 [154–225] | <0.001 |
| Length of stay (days), median [IQR] | 7 [4–11] | 3 [2–6] | 0.001 |
| Unplanned return to theatre | 11% | 9% | 1.000 |
SD, standard deviation; IQR, interquartile range.
The Western Australian Cancer Registry was interrogated a minimum 60 months following completion of treatment for each case. This facilitated collection of long-term recurrence and mortality data, which is routinely recorded by the Registry.
To compare groups undergoing parotidectomy and neck dissection against those receiving parotidectomy only, a combination of statistical analyses was employed. When outcomes were categorical with two levels, Fisher’s exact test was used with odds ratios (ORs) and confidence intervals (CIs) presented for significant results. When outcomes were continuous either an independent-groups t-test or the non-parametric Mann-Whitney U were used, depending on whether parametric assumptions had been met. Survival analyses were conducted with Kaplan-Meier curves.
Results
Demographic and clinicopathological data
A total of 522 patients were identified as undergoing “parotidectomy”, with 219 for SCC. Of these, 13 patients had insufficient data, 29 underwent parotidectomy but the parotid parenchyma was not involved with the disease, therefore 177 met inclusion criteria. Ninety-five patients with metastatic cSCC to the parotid had no clinical or radiological evidence of neck nodal metastases at initial presentation. Sixty-two patients (65%) underwent an elective neck dissection, 33 did not. The demographic and clinicopathological data for the two groups are listed in Table 1. Demographics were similar between the groups. Patients who underwent neck dissection were more likely to present with an active primary (OR =8.49, 95% CI: 1.06–68.11) and had more advanced parotid pathology (OR =2.96, 95% CI: 1.22–7.16), as measured by O’Brien P stage (1).
Management of the neck
Management of the neck varied between surgeons of different specialties, by individual surgeons and differed over the 10-year period. Of the 62 patients who underwent an elective neck dissection, 39% underwent a comprehensive neck dissection of levels I to V, the remainder underwent selective neck dissection (see Table 2). The most commonly dissected neck level was level II, followed by level III (see Table 3).
Table 2
| Neck dissection | Number of cases (n=62) | Number with neck nodal metastases (n=16) |
|---|---|---|
| II | 9 | 2 |
| III | 1 | 0 |
| I, II | 1 | 0 |
| II, III | 4 | 1 |
| II, V | 2 | 1 |
| I, II, III | 13 | 4 |
| II, III, V | 1 | 0 |
| III, IV, V | 1 | 0 |
| I, II, III, IV | 5 | 2 |
| I, II, III, V | 1 | 0 |
| I, II, III, IV, V | 24 | 6 |
Table 3
| Level | Cases with level dissected (n=62) | Level involved (n=60a) |
|---|---|---|
| Level I | 24 | 2 |
| Level II | 60 | 13 |
| Level III | 36 | 5 |
| Level IV | 25 | 2 |
| Level V | 29 | 1 |
a, levels not documented in two cases. cSCC, cutaneous squamous cell carcinoma.
Of patients who underwent a neck dissection, 75.8% had post-operative radiotherapy, either to the parotid bed or to the parotid bed and the neck. Of those who did not have surgical management of the neck, 84.8% received post-operative radiotherapy. There was no statistical difference between the groups.
Rate of occult neck metastases
The rate of occult neck metastases was 25.8%, with 16 of 62 cases that had a neck dissection having pathologically involved lymph nodes. The most commonly involved level was level II (involved in 21.7% of those with level II dissected), followed by level III (see Table 3). Of the 62 patients who underwent an elective neck dissection, we compared those with occult metastases (n=16) to those with histologically clear necks (n=46). There were no differences in demographics or clinical presentation (immunocompromise, duration of symptoms, surgical wait time, presence of active primary, O’Brien P stage or facial nerve involvement) (see Table 4).
Table 4
| Characteristics | Positive neck (n=16) | Negative neck (n=46) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 71.06±12.63 | 72.15±10.95 | 0.740 |
| Gender (males, %) | 87.5 | 78.2 | 0.490 |
| Immunocompromise (%) | 56.0 | 35.0 | 0.150 |
| Active primary (%) | 25.0 | 19.6 | 0.730 |
| P stage (2+, advanced), % | 75.0 | 52.0 | 0.150 |
| Duration of presenting complaint (weeks), median [IQR] | 8 [4–11] (n=12) | 8 [4–14] (n=40) | 0.720 |
| Facial nerve paresis (House-Brackmann 2–6) | 10% (n=10) | 11% (n=37) | 1.000 |
| Surgery wait time (days), median [IQR] | 21 [12–27] | 28 [20–47] | 0.052 |
SD, standard deviation; IQR, interquartile range; SCC, squamous cell carcinoma.
Peri-operative care
Surgical wait time was equivalent both groups. The median operative time for those who had a neck dissection was 270 minutes, compared to 169 minutes for those who had parotid surgery only (P<0.001) (see Table 1). The operations were performed in tertiary, teaching hospitals. The median length of inpatient stay was 7 days for those who underwent elective neck dissection, compared to 3 days for those in whom the neck was surgically spared (P<0.05). Unplanned return to theatre for operative management of complications was similar between the two groups.
Follow-up
The median duration of clinical follow-up post completion of treatment (either surgery or completion of post-operative radiotherapy) was 15 months (range, 0–97 months). Recurrence and survival data are routinely recorded in the Western Australian Cancer Registry allowing for collection of longer-term recurrence and mortality follow-up data, all cases were checked on the registry at 60 months post-completion of treatment.
Outcomes
The 5-year disease-free survival for patients with metastatic cSCC to the parotid without pre-operative neck nodal disease was 33.5%. Elective neck dissection did not confer a 5-year disease-free survival benefit, with no significant difference in disease free-survival at 5 years between the two groups (see Figure 1).
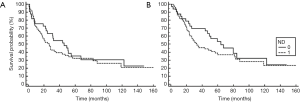
Overall survival at 5 years for the cohort was 42.5%. Neck dissection did not confer an overall 5-year survival benefit. At 5 years, 36.5% of those who underwent elective neck dissection were alive, compared to 53% of those who did not undergo neck dissection. There was no statistically significant difference in overall survival between the groups.
Discussion
This retrospective study presents a large series of patients with metastatic cSCC to the parotid treated surgically, without pre-operative evidence of neck disease. The rate of occult neck disease in 62 patients who underwent elective neck dissection in this series was 25.8%. This is the second largest Australian series of its kind, with Ebrahimi et al. reporting the largest group of cP+NO elective neck dissections, with an occult rate of 21% (9). Four Australian groups have reported rates of occult neck disease between 15% and 24% (6,10-12) with two reporting a rate of 35% (7,8). International centres have reported rates between 16% (New Zealand) (13) and 44% (USA) (4).
Authors have cited the rate of occult neck metastases as the basis for the recommendation of elective neck dissection in all patients with metastatic parotid cSCC. Dona et al. and Park et al. recommend an elective selective neck dissection in all cases (10,11). Veness et al. agreed, citing their reported 35% rate of occult metastases (7), as do Hirshoren et al. in their 2018 Australian paper that reports an occult metastases rate of 24% (12). O’Brien et al. also reported an occult rate of 35%, but did not recommend elective neck dissection, suggesting insufficient evidence to do so. Ebrahimi does not advocate elective neck dissection, but suggests their 21% rate of occult metastases contributes to the argument for elective selective neck dissection in this group. None of the aforementioned papers reported whether an elective neck dissection confers disease-free or overall survival benefit. It has been argued that an additional benefit of an elective neck dissection is that a negative neck dissection might relinquish the need for radiotherapy to the neck (8,10).
In contrast, Kirke et al. advocated against neck dissection, suggesting that “many warrant post-operative radiotherapy to the parotid bed” and that the field could be extended to include the neck (6). In their series of 51 patients with parotid cSCC, 34 underwent an elective neck dissection. In this group there was no difference in locoregional failure between those who underwent elective neck dissection and radiotherapy, compared to those whom radiotherapy was the sole treatment of the neck (6).
There was significant variability in the management of the neck in our cohort. Surgeons were more likely to perform an elective neck dissection on patients with an active primary or more advanced O’Brien P stage at presentation. It is not clear whether surgeon preference, patient preference, cancer multidisciplinary meeting consensus, or other factors contributed to the management decisions. The marked variability in neck levels dissected also further convolutes interpretation. However, when elective neck dissection is performed, taking levels II and probably III, could be considered. In this series, a neck dissection resulted in a longer time under general anaesthesia and a longer inpatient hospital stay. In this series, not all patient received post-operative radiation therapy, which further confuses interpretation of outcomes. Hirshoren et al. (2018) found that post-operative radiation therapy conferred a survival benefit.
The overall 5-year survival for this case series was 42.5%. Neck dissection did not confer any survival benefit. Given that those who underwent a neck dissection had more advanced parotid disease, one could hypothesize that the neck dissection may have been of benefit in patients with more advanced parotid pathology and/or active primary disease. Given the allocation to neck dissection was non-randomised, the absence of a difference in survival outcomes between the neck dissection and no neck dissection groups must be interpreted with caution.
Ideally, one would be able to stratify the risk of occult neck nodal metastases pre-operatively, to guide surgical management. In this series, no pre-operative demographic or clinical data was identified as predictive of neck disease and no method to identify the group of patients at risk of occult metastases has been established.
This study has limitations inherent to any retrospective study including changing clinical practice over time and inaccuracies of retrospective data collection. Despite being the second largest study of its kind identified in the literature, the sample remains underpowered and the small size limits analysis of subgroups within the cohort. The findings would be generalisable to an Australian population.
We know that patients with metastatic cSCC to the parotid have a poor prognosis, regardless of neck involvement. In those with pre-operative evidence of parotid metastasis but no evidence of neck disease, no consensus as to whether a neck dissection in this cohort of patient confers disease-free or overall survival has been reached. There continues to be a need for high-quality, prospective research to determine the best way to treat these patients given this retrospective case series confirms poor survival outcomes in this group.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Human Research Ethics Committee approval was obtained (approval number RPH HREC 15-088). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- O'Brien CJ, McNeil EB, McMahon JD, et al. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck 2002;24:417-22. [Crossref] [PubMed]
- Ch'ng S, Maitra A, Lea R, et al. Parotid metastasis--an independent prognostic factor for head and neck cutaneous squamous cell carcinoma. J Plast Reconstr Aesthet Surg 2006;59:1288-93. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer. 2017. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx
- Ying YL, Johnson JT, Myers EN. Squamous cell carcinoma of the parotid gland. Head Neck 2006;28:626-32. [Crossref] [PubMed]
- Veness MJ, Porceddu S, Palme CE, et al. Cutaneous head and neck squamous cell carcinoma metastatic to parotid and cervical lymph nodes. Head Neck 2007;29:621-31. [Crossref] [PubMed]
- Kirke DN, Porceddu S, Wallwork BD, et al. Pathologic occult neck disease in patients with metastatic cutaneous squamous cell carcinoma to the parotid. Otolaryngol Head Neck Surg 2011;144:549-51. [Crossref] [PubMed]
- Veness MJ, Morgan GJ, Palme CE, et al. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope 2005;115:870-5. [Crossref] [PubMed]
- O'Brien CJ, McNeil EB, McMahon JD, et al. Incidence of cervical node involvement in metastatic cutaneous malignancy involving the parotid gland. Head Neck 2001;23:744-8. [Crossref] [PubMed]
- Ebrahimi A, Moncrieff MD, Clark JR, et al. Predicting the pattern of regional metastases from cutaneous squamous cell carcinoma of the head and neck based on location of the primary. Head Neck 2010;32:1288-94. [Crossref] [PubMed]
- Dona E, Veness MJ, Cakir B, et al. Metastatic cutaneous squamous cell carcinoma to the parotid: the role of surgery and adjuvant radiotherapy to achieve best outcome. ANZ J Surg 2003;73:692-6. [Crossref] [PubMed]
- Park SW, Eade T, Pang L, et al. Role of neck dissection in metastatic squamous cell carcinoma to the parotid gland. J Laryngol Otol 2016;130:S54-9. [Crossref] [PubMed]
- Hirshoren N, Ruskin O, McDowell LJ, et al. Management of Parotid Metastatic Cutaneous Squamous Cell Carcinoma: Regional Recurrence Rates and Survival. Otolaryngol Head Neck Surg 2018;159:293-9. [Crossref] [PubMed]
- Ch'ng S, Maitra A, Allison RS, et al. Parotid and cervical nodal status predict prognosis for patients with head and neck metastatic cutaneous squamous cell carcinoma. J Surg Oncol 2008;98:101-5. [Crossref] [PubMed]
Cite this article as: Pollaers K, Davidoss N, Hinton-Bayre A. Management of occult neck disease in metastatic squamous cell carcinoma to the parotid gland. Aust J Otolaryngol 2019;2:24.


