
Radionecrosis of the larynx: concerns regarding surgery and reconstruction
Introduction
Laryngeal cancer is one of the most common head and neck malignancies, accounting for 20% of such cases. Most of these tumors are squamous cell carcinomas (SCC) (1,2). Up to 40% of patients present with advanced disease (3). Increased morbidity and mortality are present in a significant proportion of the advanced cases (4,5).
Radiotherapy whether primary or adjuvant, is an important line of management of head and neck malignancies. The advent of organ preservation protocols in the treatment of advanced laryngeal carcinoma, with the concomitant chemoradiotherapy being increasingly utilized, has increased the incidence of complications following radiotherapy (6-8). Radiotherapy, particularly for laryngeal cancer, causes complications in almost all patients, of variable severity, persistence and time of onset, often manifesting months or even years after the end of treatment (9-11). Radiotherapy causes alterations in the perilaryngeal and laryngeal tissues leading to local hypoxia devascularization and reduced cellular population, with subsequent inflammation and tissue fibrosis (10,11). This in turn results into laryngeal edema, radiodermatitis, perichondritis, and chondritis with necrosis (12). Chondroradionecrosis as a complication has a variable incidence, ranging from 1% to 5.3%, resulting in salvage laryngectomies in up to 25% of cases due to recurrent aspiration and loss of organ function, requiring salvage surgery, even in the absence of neoplasia (13,14). Although the timing of occurrence of chondroradionecrosis is variable, it most commonly occurs within the first year (14). Several studies show that there has been a linear relationship between the amount of radiation and the severity of tissue reaction, and consequently the development of chondroradionecrosis (5,15-17). The introduction of dose fractionation schemes and intensity-modulated radiotherapy has significantly reduced the risk of damage to normal tissues, but the risk is still present (17).
Treatment of chondroradionecrosis is problematic. Laryngeal chondroradionecrosis has been classified into 4 grades, based on the severity of symptoms, so as to guide treatment. It has been recommended that grades I and II could be managed by medical treatment and conservative measures, including humidification, hyperbaric oxygen, culture-directed antibiotics and steroids for up to 6 weeks (16,18,19).
Grades III and VI would require surgical intervention, as those patients suffer from inevitable aspiration and laryngeal dysfunction. Surgical management often inevitably leads to a total laryngectomy. Surgical options for reconstruction either to support the neopharynx or replace necrotic skin have included pedicled flaps with the deltopectoral flaps to reconstruct skin alone or more frequently using pectoralis major muscle flap alone or with skin as a myocutaneous flap (13-15,18). We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/ajo.2019.01.04)
Methods
We are presenting a case series of three patients who had received radiotherapy as a primary treatment for laryngeal cancer of different TNM stages. They were later presented with grade 3–4 chondroradionecrosis.
Results
Salvage laryngectomy was performed in all patients with addition of pectoralis major myocutaneous or myofascial flap for reconstruction as summarized in Table 1.
Table 1
| Variables | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Staging | T3N0M0 transglottic SCC | T2N1M0 Glottic SCC | T3N2M0 left Pyriform SCC |
| Co-morbidities | Hypothyroidism; hypoproteinemia | Hypertension; ischemic heart disease; hypercholesterolemia | Kidney impairment |
| Primary treatment | Radiotherapy (70 Gy over 6 weeks) | Chemoradiotherapy (cisplatinum + 60 Gy radiotherapy over 6 weeks) | Chemoradiotherapy (fluorouracil + 60 Gy radiotherapy over 6 weeks) |
| Causes of failures | Skin Necrosis (grade 4 chondroradionecrosis) | Delayed chondroradionecrosis with non-functioning larynx | Increased PET scan uptake in absence of positive biopsies |
| Laryngocutaneous fistula | Left vocal cord palsy with marked airway compromise | ||
| Salvage treatment | Total laryngectomy (6 months after treatment) | Total laryngectomy (one year after treatment) | Total laryngectomy (6 months after treatment) |
| Reconstructive option | Pectoralis major myocutaneous flap | Pectoralis major myofascial flap with split thickness skin graft | Pectoralis major myofascial flap only |
| Swallowing outcome | No fistula | Pharyngocutaneous Fistula that was managed conservatively | No fistula |
| Postoperative complication | Sepsis | Pharyngocutaneous Fistula | None |
SCC, squamous cell carcinoma.
Case one
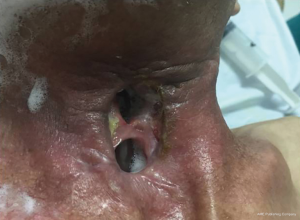
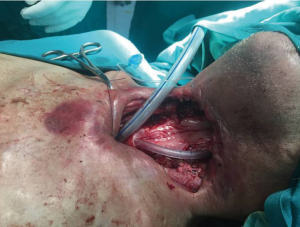
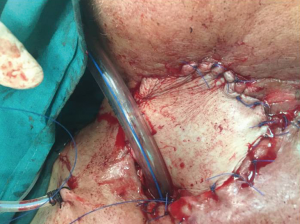
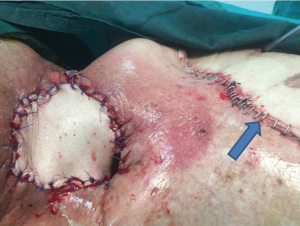
A 72-year-old diabetic ex-smoker was diagnosed with a T3N0M0 transglottic poorly differentiated SCC of his larynx. Primary treatment was conventional radiotherapy of 70 Gy for 6 weeks. Several months later he started to suffer from skin changes starting with erythema and eventually forming a laryngocutaneous fistula. He was referred to the Department of ENT-Head and Neck Surgery in Alexandria University Hospital. He was very emaciated with protein 7 g/dL and albumin 2.7 g/dL. Additionally, his prothrombin activity was 70% and he was borderline hypothyroid. There was definite aspiration for fluids and semi-solids so a nasogastric tube was inserted (readily visible through neck fistula). The patient was not tracheostomised prior to surgery. Anaesthetic team assessed his fitness as adequate for total laryngectomy and pectoralis major myocutaneous flap (PMMF). At surgery all necrotic skin and soft tissue around the larynx was excised. No lymphadenopathy was detected either pre- or intra-operatively. Extensive tissue fibrosis was noticed intra-operatively with greatly reduced bleeding from tissues and muscles (Figures 1,2). The neopharynx was closed in layers then PMMF was used to reconstruct the soft tissue and the lost skin (Figures 3,4). Oral intake was recommenced after Barium swallow excluded fistula at 10th postoperative day. Subsequent postoperative course was complicated by a severe nosocomial chest infection requiring assisted ventilation. Unfortunately, the patient died from septic shock 9 days later.
Case two
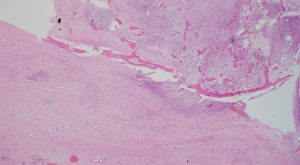
A 69-year-old ex-smoker presented to John Hunter Hospital with T2N1M0 SCC of the left glottis. Among his medical comorbidities were ischemic heart disease, type 2 diabetes mellitus, hypertension and hypercholesterolemia. He received definitive chemoradiotherapy as primary treatment. Following his treatment, he was admitted three times for conservative management of poor oral intake, nausea/vomiting, dehydration, constipation and hypokalemia. One year later, he developed a non-functioning larynx and laryngeal chondroradionecrosis was diagnosed. After presentation at the multidisciplinary team (MDT) meeting, a decision was made to perform total laryngectomy with pectoralis major flap (PMF) and left hemi-thyroidectomy. The neopharynx was closed in layers with the muscle flap overlain to support the suture line closure. A split skin graft harvested from the left thigh was placed in the skin defect over the muscle flap. On the 8th postoperative day, purulent discharge from the left neck drain was noted. Additional discharge was also seen coming from around the tracheostoma. The patient commenced on intravenous Tazocin, a cuffed tracheostomy tube was inserted into the stoma to prevent aspiration, and nasogastric tube left in place. Barium swallow demonstrated definite leak that is accumulating and compressing the upper esophagus. Subsequent CT scan revealed contrast leak from the anterior aspect of the neopharynx, so a decision was made to place a salivary bypass. After healing of the fistula, the tracheostomy tube was changed from cuffed to non-cuffed then to soft laryngectomy tube and then then discharged home on 30th postoperative day. Histology showed extensive radiation changes including fibrosis with ulceration and suppurating tissue necrosis (Figure 5).
Case three
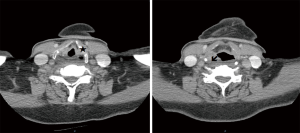
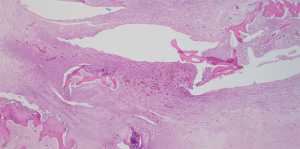
A 76-year-old ex-smoker with T3N2M0 left pyriform fossa SCC with concurrent T1 uvular SCC was treated by definitive chemoradiotherapy. He presented 6 months following completion of his chemoradiotherapy protocol with airway compromise requiring emergent awake tracheostomy. PET/CT scan demonstrated increased uptake in the left pyriform fossa and right postcricoid region (Figure 6). Multiple biopsies were taken on two separate occasions but no evidence for malignancy was found. There were no associated comorbidities apart from some impairment in renal function that improved with fluid challenge. The flexible nasolaryngoscopy findings were consistent with left vocal cord palsy. In the light of negative biopsies, a diagnosis of chondroradionecrosis was made. After discussion of the case at the Head and Neck MDT, total laryngectomy and PMF reconstruction was performed. The laryngectomy specimen demonstrated left pyriform fossa abscess cavity with suppuration and active inflammation with necrosis in the surrounding cricoid, arytenoid and thyroid cartilages (Figure 7). There was no evidence of malignancy. The postoperative period was uneventful and after contrast swallow test at postoperative day 14 the patient commenced oral feeding prior to discharge home on day 16.
Discussion
Diagnosis of laryngeal chondroradionecrosis in patients that had radiotherapy as a primary treatment for head and neck SCC is quite challenging. The main concern is to exclude the possibility of tumor recurrence. PET/CT scan can help to correlate both the metabolic activity with morphological findings. Inflammatory changes diagnosed with PET scan are often nonspecific and show significantly lower signals and different pattern when compared to cancer (20). Examination under general anesthesia with multiple tissue biopsies is another adjuvant tool, however on many occasions (case three), it was negative for necrosis or malignancy.
Treatment of laryngeal chondroradionecrosis is another challenge. In early stages (grades I and II) (18) where there are different degrees of dysphonia, odynophagia and edema of arytenoids, a variety of conservative measures like humidified oxygen, steroids and anti-reflux medications is usually helpful. Regular surveillance in outpatient clinic using fiberoptic endoscope is crucial to detect early changes suggestive of tumor recurrence (18). Uncomplicated grade III patients are usually controlled by use of systemic steroids and proper culture directed antibiotics. In 1979, Chandler (15) reported 13 cases of grade III Chondroradionecrosis that were managed conservatively with the above measures and showed complete resolution of symptoms in all 13 cases. Persistent grade III patients may require up to 6 weeks of conservative treatment (16). In such patients, hyperbaric oxygen therapy may benefit although its exact benefits remain to be proven. Ferguson et al. (19) reported treatment of four patients with grade IV laryngeal chondroradionecrosis with hyperbaric oxygen. Two patients required temporary tracheostomy, and only one patient proceeded to a total laryngectomy. The resistant grade III and grade IV (18) patients require more aggressive surgical measures and again they are considered a diagnostic challenge as it is difficult to exclude local recurrence. The functions of the larynx in such patients are severely affected so, the priority for those patients is to secure an adequate airway and prevent the complications of aspiration with possible repeated chest infections and potential local and systemic sepsis as in cases two and three. Most of grade IV laryngeal chondroradionecrosis patients are presented with life threatening problems like: severe stridor, carotid blowout, or marked soft tissue necrosis and sepsis as typically seen in case one and case three (5,17,18). Those patients are usually admitted in hospital for intravenous antibiotics, steroids, and monitoring. If there is any airway compromise, tracheotomy under local anesthesia is usually required, usually followed by direct laryngeal examination under anesthesia and biopsy to rule out tumor recurrence. Even in the absence of a conclusive biopsy like in case three, total laryngectomy is indicated by the presence of a nonfunctional, necrotic larynx. Table 2 summarizes recent studies of laryngeal chondroradionecrosis and its current management.
Table 2
| Study | Incidence of chondroradionecrosis | Primary modality of treatment | Treatment of chondroradionecrosis |
|---|---|---|---|
| Melo et al., 2017 | 10.7% | Chemoradiotherapy | Hyperbaric oxygen |
| Gessert et al., 2017 | 2.4% | Radiotherapy | Salvage surgery |
| Halkud et al., 2014 | Case series of 4 patients (2009-2013), all grade IV | Radiotherapy with one patient chemoradiotherapy for supraglottic T3N0M0 | Conservative treatment with hyperbaric oxygen |
| Roh et al., 2009 | Case series of 6 patients [2002–2007] | Either radiotherapy or chemoradiotherapy | Conservative measures with hyperbaric oxygen, salvage surgery in one patient |
Radiotherapy usually results in marked fibrosis for neck soft tissue structures even muscles like the sternocleidomastoid muscle. Skin and subcutaneous tissue show extensive damage especially in grade IV chondroradionecrosis. Reconstruction of extensive soft tissue defects using a pedicled regional flap like the pectoralis major myofascial or myocutaneous flap is usually preferred in those patients. Free flap options are less favourable due to the questionable viability of neck vessels required for anastomosis in the post-radiotherapy setting. Close monitoring and multi-disciplinary team involvement are vital in the postoperative period due to risk of post-operative complications, especially amongst those with multiple co-morbidities.
Conclusions
Chondroradionecrosis of the larynx is an uncommon but serious complication of radiotherapy. Co-morbidities such as heavy smoking, diabetes and dyslipidemia are commonly associated. Salvage resection and reconstruction using the pedicled pectoralis major myofascial or myocutaneous flap is the standard care for patients suffering from advanced and resistant grades of chondroradionecrosis.
Acknowledgments
Professor Ahmed Tantawy MD, Head of Department Otolaryngology/Head and Neck surgery, Alexandria University, Egypt for his support for head and neck cancer research and further joint activities between Egypt and Australia.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/ajo.2019.01.04
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.01.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by research ethics committee (No. 00007555). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from patients that were included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoffman HT, Karnell LH, Funk GF, et al. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg 1998;124:951-62. [Crossref] [PubMed]
- Chu EA, Kim YJ. Laryngeal cancer: diagnosis and preoperative work-up. Otolaryngol Clin North Am 2008;41:673-95. [Crossref] [PubMed]
- Shah JP, Karnell LH, Hoffman HT, et al. Patterns of care for cancer of the larynx in the United States. Arch Otolaryngol Head Neck Surg 1997;123:475-83. [Crossref] [PubMed]
- Davis GE, Schwartz SR, Veenstra DL, et al. Cost comparison of surgery vs. organ preservation for laryngeal cancer. Arch Otolaryngol Head Neck Surg 2005;131:21-6. [Crossref] [PubMed]
- Starmer HM, Tippet DC, Webster KT. Effects of laryngeal cancer on voice and swallowing. Otolaryngol Clin North Am 2008;41:793-818. [Crossref] [PubMed]
- Zbären P, Caversaccio M, Thoeny HC, et al. Radionecrosis or tumor recurrence after radiation of laryngeal and hypopharyngeal carcinomas. Otolaryngol Head Neck Surg 2006;135:838-43. [Crossref] [PubMed]
- Hillman RE, Walsh MJ, Wolf GT, et al. Functional outcomes following treatment for advanced laryngeal cancer. Part I--Voice preservation in advanced laryngeal cancer. Part II—Laryngectomy rehabilitation: the state of the art in the VA System. Research Speech-Language Pathologists. Department of Veterans Affairs Laryngeal Cancer Study Group. Ann Otol Rhinol Laryngol Suppl 1998;172:1-27. [PubMed]
- Lefebvre JL. Surgery for Laryngopharyngeal SCC in the Era of Organ Preservation. Clin Exp Otorhinolaryngol 2009;2:159-63. [Crossref] [PubMed]
- Lederman M. Radiation therapy in cancer of the larynx. JAMA 1972;221:1253-4. [Crossref] [PubMed]
- Fitzgerald PJ, Koch RJ. Delayed radionecrosis of the larynx. Am J Otolaryngol 1999;20:245-9. [Crossref] [PubMed]
- Varghese BT, Paul S, Elizabeth MI, et al. Late post radiation laryngeal chondronecrosis with pharyngooesophageal fibrosis. Indian J Cancer 2004;41:81-4. [PubMed]
- Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg 2003;129:44-9. [Crossref] [PubMed]
- Cukurova I, Cetinkaya EA. Radionecrosis of the larynx: case report and review of the literature. Acta Otorhinolaryngol Ital 2010;30:205. [PubMed]
- Oppenheimer RW, Krespi YP, Einhorn RK. Management of laryngeal radionecrosis: animal and clinical experience. Head Neck 1989;11:252-6. [Crossref] [PubMed]
- Chandler JR. Radiation fibrosis and necrosis of the larynx. Ann Otol Rhinol Laryngol 1979;88:509-14. [Crossref] [PubMed]
- Filntisis GA, Moon RE, Kraft KL, et al. Laryngeal radionecrosis and hyperbaric oxygen therapy: report of 18 cases and review of the literature. Ann Otol Rhinol Laryngol 2000;109:554-62. [Crossref] [PubMed]
- Parsons F. The effect of radiation on normal tissues in management of head and neck cancer. In: Million R, Cassisi N. editors. Management of Head and Neck Cancer: A Multidisciplinary Approach. Philadelphia: Lippincott, 1984:183-4.
- Hernando M, Hernando A, Calzas J. Laryngeal chondronecrosis following radiotherapy and concurrent chemotherapy. Acta Otorrinolaringol Esp 2008;59:509. [Crossref] [PubMed]
- Ferguson BJ, Hudson WR, Farmer JC Jr. Hyperbaric oxygen therapy for laryngeal radionecrosis. Ann Otol Rhinol Laryngol 1987;96:1-6. [Crossref] [PubMed]
- Chen YK, Su CT, Chi KH, et al. Utility of 18F-FDG PET/CT uptake patterns in Waldeyer’s Ring for differentiating benign from malignat lesions in lateral pharyngeal recess of nasopharynx. J Nucl Med 2007;48:8-14. [PubMed]
Cite this article as: Youssef A, Zahran M, Lynnhtun K, Morsy M, Domin A, Bhatia D, Cope D, Eisenberg R. Radionecrosis of the larynx: concerns regarding surgery and reconstruction. Aust J Otolaryngol 2019;2:10.


