Human papillomavirus and oropharyngeal squamous cell carcinoma: a 12-year retrospective review in a New South Wales tertiary referral centre
Introduction
Head and neck cancers are the 8th most commonly diagnosed cancers in Australia (1). Oropharyngeal squamous cell carcinoma (OPSCC) is a unique subset which has experienced a substantial increase in incidence over the last two decades (2). This rise is associated with a specific aetiological factor—the human papillomavirus (HPV), which as of 2010, was associated with 63.5% of all OPSCCs (2). This three-fold increase on figures from 1995 is mirrored in other developed countries, motivating some to declare HPV-positive OPSCC to be an epidemic (3-6).
Large overseas trials show that HPV-positive OPSCC is a clinically distinct subgroup, more likely to develop in younger patients without traditional risk factors, and associated with a better response to treatment and overall survival (7-10).
Contemporary Australian studies corroborating these findings are limited. Antonsson et al. (11) showed that the prevalence of HPV-positive disease was highest amongst non-smoking, younger patients and that HPV positivity conferred more favourable rates of one-year overall survival. Hong et al. (12) extended this to three-year survival in a study limited to tonsillar carcinoma, as well demonstrating a rising prevalence of HPV-positive OSPCC in Australia over the last 2 decades (2). These studies generally use P16INK4A (p16) immunohistochemistry (IHC), in conjunction with HPV-specific tests to define HPV positivity in their cohorts. Whilst this undoubtedly offers greater concordance with transcriptionally active HPV, it is particularly costly and there is evidence that p16 IHC alone is a reliable and cheap surrogate that can optimally stratify patient prognosis (9,13,14). Australian studies utilising this potentially more clinically appropriate measure as a standalone test are lacking in the context of oropharyngeal cancer.
This study aims to corroborate and expand upon Australian and international data on HPV-associated OPSCC, using p16 IHC to compare the clinicopathological characteristics of patients with HPV-positive and negative disease, as well as extend overall survival outcomes at all oropharyngeal subsites to three-year survival.
Methods
Study design
We performed a retrospective analysis of medical record data for patients treated at the Northern Sydney Cancer Centre (NSCC) at Royal North Shore Hospital, a large tertiary hospital in Sydney, Australia from 1st February 2005 to 3rd May 2017. The study was approved by the Human Research Ethics Committees of the Northern Sydney Local Health District ethics committees (RESP/15/218). With this approval, patient consent was not obtained due to the retrospective nature of the study.
Clinicopathological data were extracted from the head and neck cancer database at the NSCC, which maintains details of all patients treated for head and neck cancer at the centre. This database is populated through a combination of pre-consultation patient questionnaires, consultation notes and reports from subsequent investigations. We extracted information about the patient (age, sex, past medical history, smoking status, pack year history and alcohol intake), disease [histology, site and subsite, laterality, date of diagnosis, tumour (T), nodal (N), metastasis (M) and AJCC staging details], treatment (plan, intent, type and completed) and follow up (presence and date of local, regional and distant recurrence, date of death). No specific details were available regarding loss to follow up, and thus all patients’ living status was assumed to be in keeping with the entries in the ‘Current Status’ and ‘Date of Death’ database fields. Staging of patients was done according to the American Joint Committee on Cancer (AJCC), 7th edition guidelines.
Patient eligibility
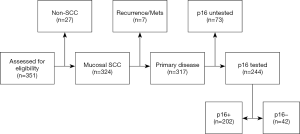
All oropharyngeal tumours which presented to the NSCC during the study period were assessed for eligibility (Figure 1). Inclusion criteria were histologically confirmed, squamous cell carcinoma of the oropharynx. Exclusion criteria were patients with recurrent or metastatic disease or patients for whom p16 status information were not present in the NSCC head and neck cancer database.
Laboratory testing
An HPV-positive tumour was defined as one testing positive on p16 IHC analysis. IHC for p16 was performed on formalin fixed paraffin embedded sections using a specific mouse monoclonal antibody (clone JC8, cat SC-56330, Santa Cruz CA, USA) at a dilution of 1 in 10. In order to reduce potential bias, results were interpreted prospectively by various attending consultant pathologists most of whom had subspecialty interest in head and neck pathology and were generally blinded to all other clinical and pathological details. Diffuse, strong, full thickness staining was categorised as p16 positive, while absent or focal weak staining was categorised as p16 negative.
Statistical analyses
Clinicopathological characteristics were compared between patients with HPV-positive and HPV-negative tumours. Time trend analyses were performed by performing a linear regression analysis to calculate slope and standard error and calculating the Pearson’s correlation coefficient. Differences between the groups were determined using a combination of independent t-tests for continuous variables, Chi-squared tests for nominal variables and the Mann-Whitney U test for ordinal variables. Survival analyses were conducted using the Kaplan-Meier method to construct time-to-event curves and calculate hazard ratios. Overall survival was defined as the time from biopsy (or if biopsy date unavailable, date of first consultation) to the date of death from any cause. Missing data was not incorporated into any statistical analysis. Analyses of clinicopathological data were conducted in SPSS Statistics 23.0 (IBM SPSS, Chicago, IL, USA), and survival analyses were performed in GraphPad Prism 7.00 (GraphPad Software, La Jolla, CA, USA). All significance tests were two-sided and a P value <0.05 represented statistical significance.
Results
Eligibility assessment
The eligibility flow chart is shown in Figure 1. A total of 351 cases of oropharyngeal tumours were assessed for eligibility; 317 were identified as OPSCC in accordance with the inclusion criteria, 244 (77.0%) of which had documented p16 testing of their tissue samples in the medical records. These patients formed the study population.
Patient characteristics
Baseline characteristics of the study population are summarised in Table 1. The mean patient age was 59.5 years (SD, 9.8) and 207 patients (84.8%) were male. Among them, 149 (61.0%) tumours were identified in the tonsil, 78 (32.0%) in the base of tongue and 17 (7.0%) in other oropharyngeal subsites. Tumours were symmetrically distributed (113 vs. 113, right-sided vs. left-sided). On questionnaire or during consultation, 162 patients (66.4%) self-identified as either current or ex-smokers and 191 (78.3%) identified as regular drinkers of alcohol. In regards to treatment, 13 (5.3%) patients received surgery alone, 13 (5.3%) received surgery and radiotherapy, 29 (11.9%) received surgery with radiotherapy and chemotherapy, 139 (57.0%) received radiotherapy with or without chemotherapy and 50 (20.5%) patient’s treatment details were unavailable.
Table 1
| Characteristic | HPV-positive (n =202) | HPV-negative (n =42) | P value | |
|---|---|---|---|---|
| Age, year | 0.005 | |||
| Mean (± SD) | 58.7 (±10.1) | 63.4 (±9.5) | ||
| Median | 62.5 | 59 | ||
| Range | 31–84 | 46–88 | ||
| Sex, No. (%) | 0.008 | |||
| Male | 177 (87.6) | 30 (71.4) | ||
| Female | 25 (12.4) | 12 (28.6) | ||
| Laterality, No. (%) | 0.127* | |||
| Left | 93 (46.0) | 20 (47.6) | ||
| Right | 101 (50.0) | 12 (28.6) | ||
| Midline | 4 (2.0) | 6 (14.3) | ||
| Bilateral | 4 (2.0) | 4 (9.5) | ||
| Subsite, No. (%) | 0.423† | |||
| Base of tongue | 69 (34.2) | 9 (21.4) | ||
| Tonsil | 126 (62.4) | 23 (54.8) | ||
| Other | 7 (3.5) | 10 (23.8) | ||
| Smoking status, No. (%) (unknown =1) | < 0.001‡ | |||
| Current | 29 (14.4) | 23 (54.8) | ||
| Ex-smoker | 93 (46.0) | 17 (40.5) | ||
| Non-smoker | 79 (39.1) | 2 (4.8) | ||
| Unknown | 1 (0.5) | 0 | ||
| Pack year history, No. (%) (unknown =52) (PYs) | < 0.001 | |||
| ≤20 | 127 (62.9) | 10 (23.8) | ||
| >20 | 34 (16.8) | 21 (50.0) | ||
| Unknown | 41 (20.3) | 11 (26.2) | ||
| Alcohol intake, No. (%) (unknown =11) | 0.318 | |||
| Drinker | 156 (77.2) | 35 (83.3) | ||
| Non-drinker | 37 (18.3) | 5 (11.9) | ||
| Unknown | 9 (4.5) | 2 (4.8) | ||
| AJCC 7th ed, No. (%) (unknown =1) | 0.068 | |||
| I | 4 (1.9) | 4 (9.5) | ||
| II | 16 (7.9) | 5 (11.9) | ||
| III | 42 (20.8) | 9 (21.4) | ||
| IV | 139 (68.8) | 24 (57.1) | ||
| Unknown | 1 (0.5) | 0 | ||
| T stage, No. (%) (unknown =2) | 0.001 | |||
| T1 | 67 (33.2) | 6 (14.3) | ||
| T2 | 83 (41.1) | 16 (38.1) | ||
| T3 | 39 (19.3) | 13 (31.0) | ||
| T4 | 11 (5.4) | 7 (16.7) | ||
| Unknown | 2 (1.0) | 0 | ||
| N stage, No. (%) | 0.001 | |||
| N0 | 26 (12.9) | 13 (31.0) | ||
| N1 | 34 (16.8) | 7 (16.7) | ||
| N2 | 133 (65.8) | 20 (47.6) | ||
| N3 | 9 (4.5) | 2 (4.8) | ||
| M stage, No. (%) | 0.261 | |||
| M0 | 195 (96.5) | 40 (95.2) | ||
| M1 | 4 (2.0) | 2 (4.8) | ||
| Mx | 3 (1.5) | 0 | ||
| Treatment, No. (%) (unknown =50) | 0.967§ | |||
| Surgery only | 11 (5.4) | 2 (4.8) | ||
| Surgery + RT | 9 (4.6) | 4 (9.5) | ||
| RT +/− CTX | 121 (59.9) | 18 (42.9) | ||
| Surgery + RT + CTX | 28 (13.9) | 1 (2.4) | ||
| Unknown | 33 (16.3) | 17 (40.4) | ||
*, left vs. right sided tumours; †, base of tongue vs. tonsil tumours; ‡, current and ex-smoker vs. non-smoker; §, surgical vs. non-surgical treatment. HPV, human papillomavirus; PYs, pack years; RT, radiotherapy; CTX, chemotherapy.
Time trend analysis of OPSCC
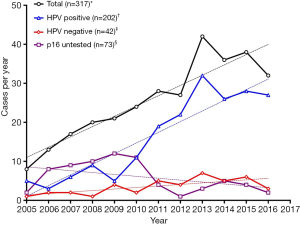
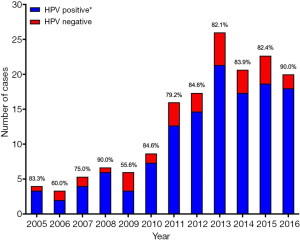
The yearly rates of OPSCC treated at the NSCC during the study are shown in Figure 2. The total number of cases per year rose significantly over the trial period (r=0.919, P<0.0001, m =2.64±0.36), despite a decrease from 2013 to 2016. The rates of HPV-positive disease increased similarly (r=0.923, P<0.0001, m =2.73±0.36) also demonstrating a fall from 2013 to 2016. HPV-negative disease rates also increased, albeit more modestly (r=0.714, P=0.0091, m =0.40±0.12). The number of untested cases per year remained constant (r=−0.453, P=0.1396, m =−0.49±0.30).
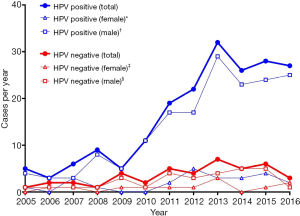
The yearly rates of OPSCC for the study population, separated by HPV status and sex are shown in Figure 3. HPV-positive disease in men rose most significantly (r=0.929, P<0.0001, m =2.47±0.31) of all the gendered subgroups, from 4 cases per year in 2005 to 29 in 2013, dropping to 25 in 2016. The number of HPV-negative cases in men showed a slight increase (r=0.616, P=0.0330, m =0.29±0.17), whilst there was no statistically significant change in number of cases per year in women, in both the HPV-positive (P=0.0556) and HPV-negative group (P=0.1487).
Proportion of HPV-positive disease
Averaging over the study period, 202 of the 244 (82.8%) patients had HPV-positive OPSCC. This proportion was higher in males than in females (85.5% vs. 67.6%, P=0.008) and was inversely related to patient age group, with the highest prevalence found in the under 50 age group (90.7%), followed by 50–59 (85.2%), 60–69 (83.3%), 70–79 (72.2%) then 80 or older (50.0%). The percentage of HPV-positive OPSCC as a proportion of all HPV tested tumours is shown in Figure 4. There was no statistically significant rise in the proportion of HPV-positive OPSCC taken as a percentage of all OPSCC (r=0.442, P=0.1506, m =1.33±0.85) over the trial period when proportions were compared on a year-to-year scale. When considered in periods of 3 years (i.e., 2005–2007, 2008–2010, 2011–2013 and 2014–2016), the proportion of HPV-positive OPSCC taken as a percentage of all OPSCC rose significantly over the trial period (r=0.99, P=0.002, m =3.87±0.17).
Clinicopathological characteristics of HPV-positive and HPV-negative OSPCC
Compared to the HPV-negative cohort, patients with HPV-positive OPSCC were significantly younger at diagnosis (mean difference =4.7 years, P=0.005) and more likely to be male (87.6% vs. 71.4%, P=0.008). Furthermore, they were significantly more likely to identify as a non-smoker (39.3% vs. 4.8%, P<0.001) and have a pack year history of 20 years or less (78.9% vs. 32.3%, P<0.001). There was no significant difference in the numbers of patients who self-identified as regular drinkers of alcohol between the two groups (P=0.318).
In the HPV-positive cohort, tumour (T) stage at presentation was less advanced (P=0.001), whist nodal (N) stage was more advanced (P=0.001). However, HPV status conferred no statistically significant difference in the metastatic (M) or overall AJCC stage at presentation. There was no statistically significant difference between the groups in terms of treatment received when treatment was classified as either surgical or non-surgical (P=0.967).
Overall survival of HPV-positive and HPV-negative OPSCC
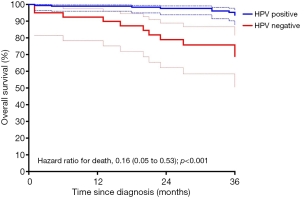
Kaplan-Meier curves for three-year overall survival are shown in Figure 5. Patients with HPV-positive disease had significantly better overall survival than those with HPV-negative disease (P<0.0001 by the log-rank test). The rates of three-year overall survival were 93.6% (95% CI, 87.9–96.6%) in the HPV-positive group and 68.9% (95% CI, 50.5–81.6%) in the HPV-negative group. The corresponding hazard ratio for death was 0.16 (95% CI, 0.05–0.53; P<0.001) favouring survival in the p16 positive group.
Discussion
This study extends survival outcome figures in an Australian cohort to three-year survival and corroborates local and international data on the clinicopathological characteristics of HPV-positive oropharyngeal cancer patients. It provides further evidence that p16 status is a significant prognosticator in the overall survival of oropharyngeal cancer patients beyond one year.
In our cohort, the increase in the cases of oropharyngeal cancer treated per year from 2005 to 2016 was largely accounted for by a rise in HPV-positive disease (Figure 2), specifically HPV-positive disease in men (Figure 3). This finding is mirrored by large, epidemiological studies both in Australia (2) and other economically developed countries (3,15). It has been suggested that geography-specific risk factors for oral HPV exposure are more prevalent in these regions (e.g., oral sex and multiple sexual partners), accounting for an increase in incidence over the last 20–30 years (15-17). This sex-specific rise has also been reported elsewhere, with HPV-positive OPSCC in women remaining at generally constant levels over the same time period in a number of other studies (3,18). Reasons for this sex predominance are unclear, but have been attributed to differences in sexual practices and a higher prevalence of HPV in cervical tissue compared to penile tissue, resulting in a greater risk of HPV transmission when performing oral sex on a female (6).
There was a small rise in HPV–negative disease over the trial period (Figure 2). This is unusual given the continuing decline in smoking rates in Australia over the last 30–40 years in Australia, however, it may in fact reflect the overall rate of population increase in the local area instead of a true rise in the prevalence of HPV negative disease.
We measured the average proportion of HPV-positive OPSCC over the study period to be 82.8%. This is somewhat higher than figures published elsewhere in Australia, which report a proportion between 49–66% (2,11,16). The discordance may reflect the relatively high socioeconomic background of the local catchment area, with research indicating that those with HPV-positive disease are more likely to occupy a higher income bracket and have a higher level of education (19). The discrepancy may also, in part, be explained by our use of p16 IHC as a standalone test to determine HPV status. This differs from other studies which combined p16 IHC with quantitative polymerase chain reaction (PCR) or in situ hybridisation (ISH) for high risk HPV as an additional criterion for HPV positivity (2,11). Pooled analysis calculate the specificity of p16 IHC alone at 83% (20) and false positive rates range from 3.8–7.3% (21) to up to 51% (22). This imprecision is an ongoing criticism of p16 testing and may result in an overestimation of the true proportion of OPSCC caused by transcriptionally active HPV, in our cohort. Other reasons for this disparity may include the inclusion of patients presenting after 2010 (thus incorporating an expected rise in prevalence of HPV-positive disease since) and the high rate of non-smokers in our cohort.
There was no statistically significant rise in the yearly proportion of HPV-positive disease over the trial period (Figure 4), however this proportion showed a significant rise when grouped in periods of three years. This is in contrast to robust epidemiological studies showing a steady rise in the yearly proportion of HPV-positive OPSCC from 2005 onwards in developed countries (2,3,18). This is likely explained by small patient numbers in the early years of our analysis, which reduces the statistical power of a time trend analysis.
HPV-positive patients were more likely to be male, present earlier, with smaller primary tumour load and more extensive nodal metastasis compared to HPV-negative patients. These findings are typical of studies comparing the two groups (23-26). Furthermore, patients with HPV-positive disease were significantly more likely to be non-smokers compared with their HPV-negative counterparts (39.3% vs. 4.8%), in keeping with previous studies (8,12). Non-smoking rates were higher both overall and in the HPV-positive cohort when compared with similar populations (2,8,11,27). Once again, this may reflect the relatively high socioeconomic status of the local catchment area. It may offer further explanation for the higher overall proportion of HPV-positive disease, reflecting a smaller carcinogenic influence of tobacco, a known risk factor for HPV-negative OPSCC.
Our survival analysis corroborates the important, previously documented finding that HPV-positive oropharyngeal cancer represents a distinct and clinically relevant subgroup with better overall survival. We calculated a hazard ratio for death of 0.16 (95% CI, 0.05–0.53, P<0.001) in favour of the HPV-positive subgroup. In previous international studies using p16 as a standalone surrogate for HPV, hazard ratios ranged from 0.21–0.49 in favour of HPV-positive OPSCC, with three-year survival rates measured as 83.6–88% in the HPV-positive group (8,9,28,29). We calculated a slightly better three-year survival rate in the HPV-positive group (91.4%), but accounting for confidence intervals, these are in keeping with international figures.
Whilst younger age and lower tobacco exposure have been offered as confounding explanations (6,30), a definitive biological mechanism accounting for the difference in survival outcomes between HPV-positive and -negative OPSCC is currently unknown. HPV-positive disease has also been shown to respond differently to treatment, appearing more chemo-radiosensitive than HPV-negative disease (31,32). Given the significant morbidity and mortality associated with current treatment regimens, this finding has supported a growing but guarded move towards de-escalated therapy for HPV-associated OPSCC. However, a 2015 Cochrane review found the current evidence base to be of insufficient quality to warrant any immediate change in treatment (33).
The major limitations of this study arise from missing data. p16 IHC results were unavailable for 23.0% of OPSCC patients excluding them from the study. It is unclear why this data was unavailable and whether it was randomly distributed. The majority of these patients presented prior to 2011 (Figure 2), likely during an era in which p16 IHC was not routinely performed on all oropharyngeal cancers (as it is today). Whilst it would have improved the accuracy of the results to have done so, financial resource limitations precluded retrospective p16 testing of tissued banked tumour samples, which would have otherwise identified the HPV status of those patients for whom p16 IHC results were not available. Furthermore, a lack of robust, quantifiable data in a number of fields restricted an investigation of the relationship between HPV status and locoregional recurrence rates, and permitted only a crude understanding of how HPV positivity is associated with pack year history, alcohol intake and treatment modality. Treatment details were unavailable for 20.5% of patients as a result of an incomplete data set. No imputation or interpolation analyses were performed. In keeping with the study’s ethics review board requirements, data prepared and released to the investigators were deidentified in a manner such that it were not possible to identify patients by name or medical record number. Correlation with patient medical records to cross-check treatment details was thus not possible due to these patient confidentiality and data deidentification standards. These standards also prohibited attempts to contact patients lost to follow up—either by corresponding directly with patients or their relatives, or by consulting public data sources such as the Registry of Births, Deaths & Marriages. These limitations detract from the findings and potentially overestimate the three-year overall survival rates, as some patients who were lost to follow up may have died in the three years following their diagnosis. In light of these constraints, it is prudent that survival data be appreciated in the broadest sense, specifically that overall survival was significantly better in the HPV-positive group, despite treatment received. Unfortunately, small numbers of patients, particularly in the early years of the study and the HPV-negative group, diminishes statistical power and the ability to draw confident conclusions from time trend analyses. Given this was a retrospective medical record review in which patients were not randomised, selection bias remains a concern in making comparisons between HPV-positive and negative patients. Finally, this study was undertaken in a single centre, limiting our knowledge of true case numbers of oropharyngeal cancer in the catchment area. As such, generalisations of the conclusions to other populations should be applied cautiously.
Since it is known that HPV-positive OPSCC represents a distinct subgroup of patients, characterised by differences in age, smoking status, T and N stage and response to treatment, some have suggested that future studies control for these variables in comparing survival outcomes (30). Other avenues of further study may include the search for precursor lesions (as in cervical cancer) and a continuing investigation into the validity of administering different treatment regimens based on HPV status, as opposed to the current paradigm in which treatment is dictated by tumour, nodal and metastatic load. Finally, the prevalence and effect of undertreating aggressive p16-positive but true HPV-negative disease when utilising a p16 standalone approach warrants investigation (34).
Of relevance, the American Joint Committee on Cancer (AJCC) released the 8th edition of the AJCC Staging Manual, Head and Neck Section intended for use with cases diagnosed from January 1, 2018. This new system reflects the paradigm shift moulded by the rising caseload and unique nature of HPV-positive OPSCC. The new edition appreciates the typical characteristics of HPV-positive patients which are identified in this and other studies (i.e., smaller tumour load, higher nodal metastasis, favourable survival) by creating a separate staging system for HPV-positive OPSCC. These HPV-positive tumours are identified by positive staining for p16 under established criteria (35). The effect of these amendments has been to correct for the skewing towards Stage III and IV disease which occurred under the 7th edition, in turn allowing clinicians to convey an AJCC stage to their HPV-positive patients that is more representative of the favourable prognosis conveyed by HPV-positivity (36). This ‘staging pitfall’ in the 7th edition was reflected in this study, which found no statistically significant difference in overall AJCC stage at presentation between the HPV-positive and HPV-negative cohorts. Similar findings have been found in previous studies which compared these cohorts under the 7th edition guidelines (8). The 8th edition now downstages HPV-positive tumours in keeping with their prognosis to more accurately reflect the reality of their natural progression. It is anticipated that the greater focus and awareness precipitated by the release of this new edition, will precipitate future HPV-positive trials in OPSCC specifically, especially given that cervical cancer, the cancer historically associated with HPV, is set to be eliminated as a public health problem in Australia within the next 20 years (37).
In conclusion, this study adds to the landscape of Australian data and corroborates international findings on HPV-associated oropharyngeal cancer. Our cohort demonstrated a higher proportion of HPV-positive disease than previous studies and confirmed that these patients are more likely to be male, present younger and identify as a non-smoker, compared to those with HPV-negative disease. Finally, our data extends Australian data on overall survival at all oropharyngeal subsites to three-year survival and suggests that p16 positivity confers a similar degree of positive prognostic value in an Australia as has been demonstrated overseas.
Acknowledgments
This manuscript was presented at the 2018 Australian Society of Otolaryngology Head and Neck Surgery Annual Scientific Meeting (ASOHNS ASM) in Perth, Australia on 9–11 March 2018.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.01.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committees of the Northern Sydney Local Health District ethics committees (RESP/15/218). With this approval, patient consent was not obtained due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Government A. Head & Neck cancer statistics. Australia. 2017. Available online: https://head-neck-cancer.canceraustralia.gov.au/statistics. Accessed 15/02/2016 2017.
- Hong A, Lee CS, Jones D, et al. Rising prevalence of human papillomavirus-related oropharyngeal cancer in Australia over the last 2 decades. Head Neck 2016;38:743-50. [Crossref] [PubMed]
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294-301. [Crossref] [PubMed]
- Hashibe M, Sturgis EM. Epidemiology of oral-cavity and oropharyngeal carcinomas: controlling a tobacco epidemic while a human papillomavirus epidemic emerges. Otolaryngol Clin North Am 2013;46:507-20. [Crossref] [PubMed]
- Lewis A, Kang R, Levine A, et al. The New Face of Head and Neck Cancer: The HPV Epidemic. Oncology (Williston Park) 2015;29:616-26. [PubMed]
- Marur S, D'Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010;11:781-9. [Crossref] [PubMed]
- Bhatia A, Burtness B. Human papillomavirus-associated oropharyngeal cancer: Defining risk groups and clinical trials. J Clin Oncol 2015;33:3243-50. [Crossref] [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [Crossref] [PubMed]
- Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4Aand human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 2010;28:4142-8. [Crossref] [PubMed]
- Rosenthal DI, Harari PM, Giralt J, et al. Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab. J Clin Oncol 2016;34:1300-8. [Crossref] [PubMed]
- Antonsson A, Neale RE, Boros S, et al. Human papillomavirus status and p16INK4A expression in patients with mucosal squamous cell carcinoma of the head and neck in Queensland, Australia. Cancer Epidemiol 2015;39:174-81. [Crossref] [PubMed]
- Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer 2013;132:2748-54. [Crossref] [PubMed]
- Lewis JS Jr. p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol 2012;6:S75-82. [Crossref] [PubMed]
- Lewis JS, Thorstad WL, Chernock RD, et al. P16 positive oropharyngeal squamous cell carcinoma: An entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol 2010;34:1088-96. [Crossref] [PubMed]
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 2013;31:4550-9. [Crossref] [PubMed]
- Hong AM, Grulich AE, Jones D, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine 2010;28:3269-72. [Crossref] [PubMed]
- Rosenquist K, Wennerberg J, Schildt EB, et al. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol 2005;125:1327-36. [Crossref] [PubMed]
- Forte T, Niu J, Lockwood GA, et al. Incidence trends in head and neck cancers and human papillomavirus (HPV)-associated oropharyngeal cancer in Canada, 1992-2009. Cancer Causes Control 2012;23:1343-8. [Crossref] [PubMed]
- Dahlstrom KR, Bell D, Hanby D, et al. Socioeconomic characteristics of patients with oropharyngeal carcinoma according to tumor HPV status, patient smoking status, and sexual behavior. Oral Oncol 2015;51:832-8. [Crossref] [PubMed]
- Prigge ES, Arbyn M, von Knebel Doeberitz M, et al. Diagnostic accuracy of p16INK4a immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int J Cancer 2017;140:1186-98. [Crossref] [PubMed]
- Seiwert T. Accurate HPV testing: A requirement for precision medicine for head and neck cancer. Ann Oncol 2013;24:2711-3. [Crossref] [PubMed]
- Wang H, Sun R, Lin H, et al. P16INK4A as a surrogate biomarker for human papillomavirus-associated oropharyngeal carcinoma: consideration of some aspects. Cancer Sci 2013;104:1553-9. [Crossref] [PubMed]
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000;92:709-20. [Crossref] [PubMed]
- Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008;100:407-20. [Crossref] [PubMed]
- Näsman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int J Cancer 2009;125:362-6. [Crossref] [PubMed]
- Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck 2008;30:898-903. [Crossref] [PubMed]
- Wells LAR, Junor EJ, Conn B, et al. Population-based p16 and HPV positivity rates in oropharyngeal cancer in Southeast Scotland. J Clin Pathol 2015;68:849-52. [Crossref] [PubMed]
- Weinberger PM, Yu Z, Haffty BG, et al. Prognostic significance of p16 protein levels in oropharyngeal squamous cell cancer. Clin Cancer Res 2004;10:5684-91. [Crossref] [PubMed]
- Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol 2009;27:6213-21. [Crossref] [PubMed]
- O'Rorke MA, Ellison MV, Murray LJ, et al. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol 2012;48:1191-201. [Crossref] [PubMed]
- Hong AM, Dobbins TA, Lee CS, et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer 2010;103:1510-7. [Crossref] [PubMed]
- Lindel K, Beer KT, Laissue J, et al. Human papillomavirus positive squamous cell carcinoma of the oropharynx: A radiosensitive subgroup of head and neck carcinoma. Cancer 2001;92:805-13. [Crossref] [PubMed]
- Masterson L, Moualed D, Masood A, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cochrane Database Syst Rev 2014;2:CD010271. [PubMed]
- Jouhi L, Hagström J, Atula T, et al. Is p16 an adequate surrogate for human papillomavirus status determination? Curr Opin Otolaryngol Head Neck Surg. 2017;25:108-12. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. editors. AJCC Cancer Staging Manual. 8th ed. New York, 2017.
- Lydiatt WM, Patel SG, O'Sullivan B, et al. Head and Neck cancers—major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:122-37.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health 2019;4:e19-e27. [PubMed]
Cite this article as: Taylor A, Eade T, Veivers D, Gill AJ, Pang L. Human papillomavirus and oropharyngeal squamous cell carcinoma: a 12-year retrospective review in a New South Wales tertiary referral centre. Aust J Otolaryngol 2019;2:1.