
The assessment of pulsatile tinnitus—a systematic review of underlying pathologies and modern diagnostic approaches
Introduction
Vascular pulsatile tinnitus (PT) is an auditory phenomenon that occurs in the absence of an external stimulus, and presents as a humming or whooshing sound, in synchrony with the subject’s heartbeat (1,2). PT can be subjective, only perceived by the patient, or objective, audible to both patient and a third party (3), and unilateral or bilateral, though generally presents asymmetrically. Cases of PT have been associated with a variety of underlying causes, ranging from the benign and spontaneously resolving to the debilitating and potentially life threatening (2,4). Given this variability in presentation and pathology it is crucial that approaches to diagnosis are consistent.
This report aims to (I) provide a comprehensive review of the most common causes and methods of assessment of PT, with the goal of (II) developing an updated set of diagnostic guidelines. These recommendations should be practical, effective, and balanced with respect to managing the risks of misdiagnosis against the often-overlooked cost to a patient directed to commence potentially unnecessary imaging studies.
The most common causes for PT are venous and arterial anomalies such as dural arterial venous fistulae, aberrant internal carotid arteries, benign intracranial hypertension (BIH), and certain middle ear masses including glomus tumours and dehiscent jugular bulbs (4). We describe the characteristic clinical and radiological findings related to each of these pathologies, and provide representative radiographs of each condition for the reader’s reference.
CT was the preferred imaging technique for most PT case studies. Contrary to this practice, we make an argument for prioritising magnetic resonance imaging (MRI), citing its superior sensitivity and the lower associated morbidity. We concede that there remain some cases wherein both MRI and CT must be employed to provide an accurate diagnosis, and we outline these circumstances. We acknowledge also that angiography may be used in combination with either of these techniques to provide a more comprehensive overview of pathology-related vasculature and to inform surgical approaches, should surgical intervention be deemed appropriate. We argue that MRI, CT, and angiography studies can constitute a significant financial burden and medical risk to the patient. Therefore, we suggest foregoing an extensive and expensive imaging study in those cases where a thorough medical history and physical examination do not indicate a malicious cause.
Methods
Eligibility criteria
Our aim in this study was to review the causes of PT and current trends in diagnostic radiology for patients with these conditions. Our goal was to evaluate coherence between institutional protocols, retrospective reviews, and one-off case studies. Patient demographics, aetiologies and natural histories of the cases were not restricted during our search, though these features were later used to group and analyse results.
Information sources
We reviewed a collection of articles sourced from the online database, PubMed, using the search term “pulsatile tinnitus” and applying the following filter terms: [“imaging”, “MRI”, “CT”], [“diagnosis”, “causes”, “aetiology”] and [“diagnosis”, “subjective”, “objective”]. All results used in the final version of this text were collected between June and July 2018.
Study selection
Results were sorted to remove duplicates where the same text was produced by multiple searches, then filtered by language of publication (English texts only), and relevance of content (texts were excluded if, for example, “pulsatile tinnitus” was absent from the publication’s keywords list). All 50 texts in the final selection had at least 10 citations in the NCBI database; the maximum number held by a single text was 184. The final selection included a minority group of pre-2000 texts—1 article published in 1979, 3 articles published between 1980–1989 and 11 articles published between 1990–1999. After careful consideration, we decided to retain these older documents to facilitate a broad analysis of changing trends over time. A modern perspective was successfully encapsulated in our search results with nearly half of our filtered collection published in the last decade, and 12 in the last 5 years.
Where necessary, the articles sourced as described above were supplemented with a small selection of highly cited papers relevant to the discussion of particular points in this review.
Data collection
Data were extracted from reports independently and directly from in-text content. All initial mining was done by the first author (KE Grierson), who has training in literature review and criticism. Initial assessment was confirmed by a consulting scientist with a professional background in hearing research. All technical questions and disagreements between the two assessors were resolved by discussion with second and third authors. No information needed clarification by original authors for the purpose of our investigation. The information obtained from each article depended on the type of publication—case study, retrospective review, or technical report—but generally included definitions of pathologies presented; gender ratios and age ranges of patients; PT laterality, duration, and comorbidities; type, order, and rationale for imaging modalities used in diagnosis; and any indicators of imaging sensitivity.
Data summaries and synthesis
Our ability to effectively consolidate and summarise data was limited by three implicit features of our information sources:
- Methods of data collection varied significantly between articles;
- Even after pooling, sample sizes were relatively small;
- Patient types and approaches to treatment depended heavily on the institute and department (e.g., an audiologist was more likely to report on an extensive audiometric and physical exam compared to a consulting neurosurgeon) (5).
To overcome these limitations, consistent parameters, such as age and PT sidedness, have been simplified as percentages over the pooled population of “PT patients”, grouped into data from case studies and retrospective reviews, separately. More complex parameters are dealt with individually by study. Comments on sensitivity of different imaging modalities are given as reported in original texts, where those data were available. Sensitivity was not estimated numerically in many articles. For those cases, we grouped articles by choice of primary imaging modality and evaluated the diagnostic potential of each approach with reference to secondary imaging, presenting symptoms and conclusive findings, i.e., given a prescribed set of symptoms, how likely is a given imaging approach going to detect the underlying pathology? Data are given in Tables 1-3.
Table 1
| Publication details (author, year) | Demographics | PT presentation | Comorbidities & risk factors | Diagnostics | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (years) | Lat. | Duration (months) | H | HL | O | Ot | Ob | B | AF | V | P | HI | C | R | OH | Primary imaging modality | Initial finding | Conclusive finding | ||||
| Bitoh, 1979 | M | 28 | Right | 3 | X | X-ray | Irregular linear fracture near right transverse sinus | DAVM | |||||||||||||||
| Gruber, 1991 | F | 44 | Left | Long term | X | Doppler flow ultrasound | Normal | Fibromuscular dysplasia | |||||||||||||||
| Mehall, 1993 | M | 36 | Right | 1.5 | * | CT | Abnormality near right mastoid sinus | Laterally placed, tortuous sigmoid sinus | |||||||||||||||
| Piepgras, 1994 | F | 55 | Left | 6 | X | CT | Small hyperdense lesion adjacent to the right sylvian fissure | Aneurysm due to intracranial dissection of distal middle cerebral artery | |||||||||||||||
| Pirodda, 1994 | F | 26 | Left | 6 | X | X | CT | Intratympanic mass (suspected glomus tumour) | Aberrant ICA, persistent stapedial artery, thickened stapedial footplate | ||||||||||||||
| Adelman, 1998 | M | 21 | Left | 4 | X | X | X | X | CT and MRI | IIP | DAVM and related IIP | ||||||||||||
| Reece, 1999 | M | 21 | Left | Acute onset | X | X | X | CT& MRI (contrast enhanced) | Middle ear tumour | Aneurysm of petrous ICA | |||||||||||||
| Rosenbloom, 1999 | F | 33 | Right | 1.5 | X | X | X | X | X | CT | Skull base tumour | Giant cell tumour | |||||||||||
| Houdart, 2000 | F | 33 | Left | 6 | X | X | CT | Smooth cavity in left temp bone | Aneurysm of dural sigmoid sinus | ||||||||||||||
| Pak, 2001 | F | 59 | Left | 6 | X | CT | Laterally displaced ICA | Lateralized ICA | |||||||||||||||
| Tuz, 2003 | M | 71 | Left | 36 | X | CT | Extensive pneumatization of temp bone | Pneumatization of the temporal bone around ICA | |||||||||||||||
| Xenellis, 2005 | F | 28 | Left | NA—unreported | X | X | CT | Epidural pneumocephalus in the left posterior fossa | Epidural pneumocephalus in left posterior fossa | ||||||||||||||
| Endo, 2006 | F | 37 | Right | 5 | X | X | MRI/MRA | Severe stenosis of ICA | Aberrant ICA | ||||||||||||||
| Mehanna, 2010 | F | 46 | Right | 72 | X | X | X | X | MRI/A | Normal | High riding jugular bulb, venous aneurysm diverticulum → dural venous sinus aneurysm | ||||||||||||
| Pons, 2012 | M | 43 | Left | 24 | X | CTA | Left sigmoid sinus dilation, emissary vein ectasis | Sigmoid sinus thrombosis → emissary vein ectasis | |||||||||||||||
| Chang, 2013 | M | 68 | Right | 1 | X | X | Duplex Ultrasound | Normal | NVCC → paroxysmal PT and vertigo | ||||||||||||||
| Lee, 2013 | F | 44 | Left | 6 | X | X | CT (contrast-enhanced) | Left MEV showing prominent opening to sigmoid sinus | Single dilated MEV | ||||||||||||||
| Santa Maria, 2013 | F | 24 | Right | 48 | X | X | CT | Large, lateral sigmoid sinus, SSDD | SSD | ||||||||||||||
*, Mehall 1993 report incomplete examination; no compression test performed. Based on diagnosis, this test should have given a positive result. H, headache; HL, hearing loss; O, otalgia; Ot, otorrhagia; Ob, objective pulsatile tinnitus; B, bruit; AF, aural fullness; V, vertigo; P, papilledema; HI, head injury/trauma; VD, vascular disease; C, PT changes with neck compression or Valsalva manoeuvre; R, retrotympanic mass; OH, other history; MEV, mastoid emissary vein; ICA, internal carotid artery; SSD, sigmoid sinus diverticulum; SSDD, sigmoid sinus dehiscence; IIP, increased intracranial pressure; BIH, benign intracranial hypertension; NVCC, neurovascular cross compression; DAVM, dural arteriovenous malformation; DAVF, dural arteriovenous fistula; Lat., laterality.
Table 2
| Publication details (author, year) | # PT cases | Demographics | PT presentation | Comorbidities & risk factors | Diagnostics | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/F ratio | Age range; average | L/R/bilateral | H | HL | O | Ot | Ob | B | AF | V | P | HI | C | R | OH | Primary imaging modality | Conclusive findings | |||||
| Baomin, 2014 | 46 | 44/2 | 22–58; 42.1 | 12/34/2 | X | X | MRV and DSA | DVS stenosis | ||||||||||||||
| Daneshi, 2004 | 34 | 14/20 | 17–80; 34 | 31/3b | X | X | Doppler flow ultrasound | ACAD | ||||||||||||||
| De Ridder, 2005 | 52 | 24/28 | 14–80; 49.7 | 17/35b | X | X | X | X | MRI | Intrameatal vascular loop | ||||||||||||
| Dietz, 1994 | 49 | 17/32 | 12–80; 48 | 49/24/4 | X | X | X | X | X | X | X | X | X | MRI | Aberrant ICA, DAVF, glomus tumour, sinus abnormality, JB abnormality, idiopathic | |||||||
| Friedmann, 2010 | 11 | 6/5 | 5–82; 46.7 | 6/5/0 | X | X | X | CT | JB abnormality | |||||||||||||
| Grewal, 2014 | 15 | 0/15 | 26–73; 45 | 4/8/3 | X | X | X | X | X | X | CT | SSD/SSDD | ||||||||||
| Herraiz, 2007 | 80 | NA | NA | NA | X | X | X | X | MRI and Doppler flow ultrasound | BIH, aberrant ICA, otitis media, DAVF, JB abnormality, intrameatal loop, high cardiac output state, idiopathic, other | ||||||||||||
| Hofmann, 2013 | 77 | 26/51 | NA; 56 | 27/38/12 | X | X | X | X | X | X | CT/CTA and MRI/MRA | Glomus tumour, DAVF/DAVM, BIH, arterial stenosis, SSD, anatomical variants, idiopathic, capillary hyperemia, other | ||||||||||
| Liu, 2015 | 33 | 5/28 | NA; 33.97 | 4/29/0* | X | X | X | MRI | SSD/SSDD, BIH | |||||||||||||
| Mundada, 2015 | 32 | 12/20 | 15–74; NA | 13/18/1 | X | X | CTA/CTV | Aberrant ICA/ICA aneurysm, SSD/SSDD, JB abnormality, intrameatal vascular loop, DAVF/DAVM, glomus tumour, BIH, fibromuscular dysplasia, idiopathic, other | ||||||||||||||
| Otto, 2006 | 5 | 2/3 | 49–68; 59 | 3/1/1 | X | CTA | SSD/SSDD | |||||||||||||||
| Pelkonen, 2004 | 16 | 7/9 | 33–55; 43.8 | 6/7/3 | X | X | X | CT and MRI | Arterial dissection | |||||||||||||
| Shin, 2000 | 54 | 22/32 | 34–77; 52.8 | NA | X | X | X | X | CT and MRI/MRA | DAVF, narrow transverse sinus, fibromuscular dysplasia, carotid cavernous/vertebral fistula, subarachnoid hemorrhage, idiopathic | ||||||||||||
| Shweel, 2013 | 27 | 16/11 | 23–85; 48 | 18/13 (4 bilateral) | X | X | MRI/MRA | DAVM, JB abnormalities, ICA aneurysm, vertebral artery hypoplasia, glomus tumour, idiopathic | ||||||||||||||
| Sila, 1986 | 20 | 5/15 | 26–79; 54.65 | 10/5/5 | X | X | DSA | DAVM, ICA occlusion, carotid stenosis, sinus thrombosis, arterial ectasis, arterial dissection, idiopathic | ||||||||||||||
| Sismanis, 1994 | 12 | 4/8 | 50–82; 66 | 5/6/1 | X | X | CT and MRI | ACAD | ||||||||||||||
| Sonmez, 2007 | 74 | 44/30 | 5–87; 42.8 | 28/40 (12 bilateral) | X | X | HRCT | JB abnormality, jugular diverticulum, glomus tumour, DAVF, aberrant ICA/ICA aneurysm, idiopathic | ||||||||||||||
| Wang, 2015 | 28 | 2/26 | 27–46; NA | 10/18/0 | X | X | X | CTA/CTV | SSD/SSDD | |||||||||||||
| Xue, 2012 | 3 | 0/3 | 30–54; 40 | 2/1/0 | X | CTA/V | Focal defect of the mastoid bone shell | |||||||||||||||
H, headache; HL, hearing loss; O, otalgia; Ot, otorrhagia; Ob, objective pulsatile tinnitus; B, bruit; AF, aural fullness; V, vertigo; P, papilledema; HI, head injury/trauma; C, PT changes with neck compression or Valsalva manoeuvre; R, retrotympanic mass; OH, other history; PT, pulsatile tinnitus; MRV, magnetic resonance venography; DSA, digital subtraction angiography; ICA, internal carotid artery; DAVF, dural arterial-venous fistula; SSD, sigmoid sinus diverticulum; SSDD, sigmoid sinus dehiscence; BIH, benign intracranial hypertension; DAVM, dural arteriovenous malformation; MRA, magnetic resonance angiography; CTA, computed tomographic angiography; CTV, computed tomographic venography; HRCT, high-resolution computed tomography; ACAD, atherosclerotic arterial disease; DVS, dural venous stenosis; JB, Jugular bulb.
Table 3
| Study type | Demographics | PT presentation | Comorbidities & risk factors (%) | Diagnostics | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Laterality | Duration | H | HL | O | Ot | Ob | B | AF | V | P | HI | C | R | OH | Primary imaging modality | |||||
| Case studies | M—38.9% | Rng: 21–71 years | Right—38.9% | Rng: 1 month—6 years | 5.6 | 33.3 | 16.7 | 5.6 | 5.6 | 33.3 | 5.6 | 5.6 | 5.6 | 11.1 | 33.3 | 5.6 | 27.8 | CT—55.6% (n=10) | CT/MRI—11.1% (n=2) | |||
| F—61.1% | Av: 39.8 years | Left—61.1% | Av: 1 year 3 months | MRI—11.1% (n=2) | Other—22.2% (n=4) | |||||||||||||||||
| Reviews | M—42.6% | Rng: 5–87 years | Right—51.5%; Bilat.—8.5% | – | 36.8 | 31.6 | 0.0 | 0.0 | 26.3 | 26.3 | 15.8 | 31.6 | 0.0 | 26.3 | 21.1 | 15.8 | 84.2 | CT—36.7% (n=7) | CT/MRI—21.1% (n=4) | |||
| F—57.4% | – | Left—40.0% | – | MRI—21.1% (n=4) | Other—21.1% (n=4) | |||||||||||||||||
Rng, range; Av, average; Bilat., bilateral; H, headache; HL, hearing loss; O, otalgia; Ot, otorrhagia; Ob, objective pulsatile tinnitus; B, bruit; AF, aural fullness; V, vertigo; P, papilledema; HI, head injury/trauma; C, PT changes with neck compression or Valsalva manoeuvre; R, retrotympanic mass; OH, other history; PT, pulsatile tinnitus; CT, computed tomography; MRI, magnetic resonance imaging.
Epidemiological data, such as rates of appearance in a population or dominance of a particular symptom for a given pathology, are presented as ranges using reported maximum and minimum values and upper and lower limits as given in texts. For case studies, data are given based on individual cases; for retrospective reviews, data are given as representative for the cohort (i.e., one unit of value represents one text).
Qualitative data, such as medical definitions, are described using language taken from relevant articles. Direct quotations are referenced appropriately. Where data were missing or incomplete, they have been reported as “N.A.” in the summary tables, and removed from the analysis for that category.
Biases
No biases were observed within studies, other than a tendency of individual practitioners to favour the imaging protocol laid out by their institutional guidelines. As this pattern was informative for our investigation, we chose to accept these varying diagnostic approaches, and loosely interpreted decision-making within individual studies as representing institutional practice.
Results
There exist several clinically detectable causes of PT
The most striking finding to come out of this review is the expansive breadth and depth of pathologies that are causal-for, or at least common correlates-of PT. These conditions range from the benign to the life threatening, and stem from genetic defects, chronic diseases, and/or acute injuries. Tables 1-3 summarise the types and frequencies of PT-related conditions identified in our reading. Generally speaking, a glomus tumour or similar will be visible in the middle ear (Table 4) (2,6,7), while auscultation of the scalp, neck and eyes will often indicate that some form of arterio-venous fistula is present (8). PT in a young, obese, commonly female patient will lead to a suspicion of BIH (4). A hyperdynamic circulatory state (thyrotoxicosis, fever, widespread Paget’s disease, etc., Table S1) can be suspected after a physical examination (9). There remains a group of patients for whom no diagnosis is suggested by either the history or the clinical examination and although, in theory, these may have a detectable underlying cause, many are truly idiopathic.
Table 4
| Common middle ear masses |
| Paraganglioma (glomus) |
| Meningioma |
| Adenomatous neoplasm |
| Herniated brain |
| Aberrant internal carotid artery |
| High (giant) jugular bulb |
The middle ear
The most common middle ear mass to produce PT is a glomus tumour (paraganglioma) (10,11). Clinical examination can reveal a reddish-blue mass filling the lower half of the middle ear cavity (8,12). A clinical exam will also detect an aberrant internal carotid artery (ICA) lying in the antero-inferior quadrant of the tympanic cavity (6,13). A high and dominant jugular bulb will be seen posteroinferiorly and has a blueish appearance (14). Both bone window CT and MRI give significant information about all of these conditions, wherein biopsy is not necessary or recommended. Common middle ear masses are listed in Table 4.
Arterial abnormalities
Every patient with PT should be subject to auscultation (preferably in a sound proof booth) focusing on the mastoid area, the orbits, and the anterior neck (5,15). A positive result indicates that a life-threatening condition could be present. These high-risk conditions include a dural arterial-venous fistula (DAVF) (8,16), a carotico-cavernous fistula (17), carotid artery atherosclerosis (2,10), and fibro-muscular dysplasia (5,10), the latter being most commonly seen in middle-aged women (4). When a bruit is present, further investigation is mandatory.
Venous causes
Suggested causes of venous PT include a high riding jugular bulb, sigmoid sinus arachnoid granulation, abnormal condylar or mastoid emissary veins, and abnormal venous collaterals (3,5,18-20). These latter conditions have been found in conjunction with venous PT, but a cause and effect relationship has not been established. The natural history of these conditions is not one associated with morbidity.
BIH
BIH occurs mostly in young obese females. As we see an increase in overweight and obese adolescents in the Australian population (21), incidence of BIH is expected to rise, and with it, the importance of understanding this diagnosis. In cases of BIH, PT can be accompanied by aural fullness, hearing loss, dizziness, headaches and visual disturbances. Often, the PT will increase with compression of the contralateral jugular vein and decrease with compression of the ipsilateral jugular vein. Fundoscopy can indicate early fundus changes or papilloedema (22). There is a strong incidence of serious visual problems in this condition (4,5). MRI is indicated with the common findings listed in Table S2. Lumbar puncture is usually not necessary if the clinical and MRI findings are suggestive of BIH (4).
Rare associations
Conditions less commonly reported in association with PT include a persistent primitive trigeminal artery (23), meningocele of the temporal bone (9) and a focal defect in the mastoid bone shell adjacent to the transverse-sigmoid junction (24). A dehiscent superior semicircular canal has also been reported during an investigation of objective PT (4). Myoclonic contractions of the middle ear muscles have been linked to a type pf PT, although this is a rare pathology (4). Moreover, this phenomenon is not a pulse-synchronous tinnitus, and therefore outside of the scope of this review. Finally, PT is a common symptom in patients with Von Hippel-Lindau Disease (VHL), but only in cases where an endolymphatic sac tumour is the cause of the pathology (25). In these cases, concurrent presentation of vertigo and hearing loss should prompt the clinician to proceed to investigation.
PT has led on occasion to the diagnosis, probably as a chance finding, of intracranial aneurysm (Kim 2011) or aneurysm of the ICA (9). However, an aneurysm presenting as PT is extremely rare (9) where the lesion is generally very large and is accompanied by headache or high blood pressure (26).
MRI is the most sensitive imaging modality for diagnosing PT
If imaging is indicated, ideally it will be cost-effective, comprehensive, non-invasive and radiation free. MRI is best suited for this and over the last decade has become far more accessible. Cost remains higher than CT, though its superior contrast resolution and sensitivity for a wider spectrum of pathology justifies this difference. MRI with contrast can often be the sole imaging investigation.
Imaging should be directed by clinical findings and medical history
A thorough understanding of the various etiologies associated with PT is invaluable for the clinician involved in the initial assessment of a PT patient. A through physical examination and comprehensive medical history will direct the clinician to a specific hypothesis, facilitating the choice of an appropriate approach to diagnostic imaging (11).
In taking the medical history, it is important to note risk factors including head trauma, tumours, and any cardiovascular issues, e.g., coronary artery disease or hypertension, comorbidities such as vertigo, hearing loss, aural fullness, headache or neck ache, and any observable fluctuations in the PT (5,12,27-29). Does the PT worsen after exercise or when lying down? The patient should be asked to describe the perceived intensity of the PT, and an inquiry should be made as to the specific reason for their visit. That is, has the intensity of the symptom acutely changed, does the symptom seem to be gradually worsening, or has a new symptom recently developed?
Initial physical examination should include otoscopy to assess the appearance of the tympanic membrane and to note the presence of a post auricular mass (1,2,5,30); also, auscultation of the scalp, neck and eyes is necessary to rule-out a serious arterial pathology such as arterio-venous fistula (5,11,15,31). Ideally, auscultation should be done in a soundproof booth. Blood pressure should be checked, and tests for a high cardiac output state should be ordered (8,32,33). Audiometry should be performed to quantify any conductive or sensorineural hearing loss (15,34,35). Compression of the jugular vein by gentle pressure on the neck and/or use of the Valsalva maneuver to reduce venous return will differentiate between an arterial or venous origin (5,27,32,36); improvement of the symptoms during either of these procedures points to a venous cause on the ipsilateral side. Fundoscopy will detect papilloedema and establish suspicion of BIH (5).
Modern MRI is sensitive to most PT-related pathologies
Clinical findings in PT patients should direct approaches to diagnostic radiography. However, even with thorough clinical data, clinicians may still be left considering multiple options for the primary imaging study in a given case.
Cerebral angiography remains the gold standard for diagnosis of PT-related vascular pathologies (11). However, this procedure is invasive and has a high risk of morbidity compared to other available imaging modalities. Angiography should therefore be reserved for secondary imaging to confirm a diagnosis or to facilitate pre-surgical preparation. Since the 1980’s, CT has been favoured as the primary imaging modality for PT patients (37). In our literature selection, CT alone is the preferred imaging modality in 11/18 case studies (16,18,27,28,31,38-43), and CT is used in parallel with MRI for initial investigations in another 2/18 (44,45). However, extensive CT imaging results in prolonged patient exposure to radiation. Furthermore, stand-alone CT is rarely sufficient for an accurate diagnosis, and follow-up MRI/magnetic resonance angiography (MRA), Doppler ultrasonography, and/or digital subtraction angiography (DSA) are usually required for confirmation and to extract more detail about a given lesion (16,18,38-40).
The authors propose that MRI should be considered as the default imaging modality in diagnosing most cases of PT. Advances in the biomedical sector over the last two decades have seen significant improvements in MRI technology. MRI is now both reliably sensitive to most PT-related pathologies and accessible to common patients (46). Modern MRI has been found to accurately detect the following: dural venous sinus stenosis, a laterally-placed/tortuous sigmoid sinus, aberrant/lateralised carotid artery, dilated mastoid emissary vein, glomus tumour, sigmoid sinus dehiscence or diverticulum, intrameatal vascular loops, arterial dissection, and a dural-arterial venous fistula/malformation (47-51). Supplementing MRI with MRA has been shown to significantly increase sensitivity of the diagnosis (37). MRI is widely recognised as a safer, less invasive procedure than CT or traditional angiography, and the disparity of costing between MRI and the less expensive CT has been significantly reduced in recent years (52,53). The authors argue that any remaining difference in costing is justified by the greater utility of MRI in the accurate diagnosis of PT.
There are exceptions to the MRI recommendation
Where MRI is contraindicated, such as in patients with non-MR compatible cardiac devices or cochlear implants (41) a non-contrast CT brain with petrous temporal bone views can be performed as the basic screening protocol. A more comprehensive study with contrast enhanced CT, cerebral angiogram and venogram can be performed, albeit with greater radiation exposure.
Colour-flow Doppler imaging provides a reliable non-invasive approach where an arterial dissection, particularly a dissected carotid artery, is strongly suspected (5,54). These findings can be confirmed with diffusion-weighted MRI if necessary.
There remain some vascular anomalies, such as fistula of a semicircular canal, which are best detected with a non-contrast CT of the petrous temporal bone (6). However, the presence of a definitive cause-effect relationship in these pathologies remains yet to be confirmed.
Finally, an argument can be made for foregoing imaging in some cases of PT. Despite the effectiveness of modern radiological methods, CT, MRI and angiography are associated with a variety of medical risks, and present a significant financial burden (52,53). An MRI scan may be uncomfortable due to the confined space of the MRI chamber and loud noises generated by the machine (55). CT involves exposure to ionising radiation, and though a single scan will provide a low-level dose, repeat scans may have a cumulative effect (56). Contrast dyes may trigger an allergic reaction or have negative effects on kidney function (55). Angiography, as previously stated, involves catheterisation. This procedure requires application of local anaesthesia and possible sedation, both of which carry a small risk of allergic reaction (57). Catheterisation can also result in bruising or bleeding (57). MRI, CT and angiography are not recommended for pregnant women. Furthermore, diagnostic radiography of the cranial and neck regions in Australia can cost from AUD$160 to over AUD$400, depending on the modality and parameters of the scan (53,58). Therefore, use of these techniques should be reserved for cases in which a diagnosis is likely and/or where a dangerous underlying pathology is reasonably suspected.
Often in large clinical reviews, there remains a small minority of subjects for whom no underlying pathology can be identified (3,59,60). In Herraiz 2007 (5), 10/80 cases were designated “idiopathic” by the completion of the study; in Dietz 1994 (37), 21/49 cases had no demonstrable cause for their PT; and Sonmez 2007 (6) reported 24 of 74 total cases, or 38.1% of the subjective cases, as having “normal” radiological findings. No negative outcomes have been reported for any of these cases. Idiopathic PT patients almost exclusively present with subjective symptoms, and there is rarely a risk factor highlighted in the medical history of these cases that would lead to suspicion of a dangerous pathology. It has been proposed that in at least some these, the tinnitus may be caused by natural anatomical variations (60).
Only one PT case in our entire series presented with “no” risk factors before imaging found an underlying pathology (38). However, the case report stated that the physical examination was not complete—there was no neck compression test performed or use of the Valsalva maneuver. Given that the conclusive finding in this case was an abnormal cranial venous sinus, a complete physical examination would have provided a positive indication for the clinician. There were no other reports of a pathological finding without an identified risk factor. Evidence for major pathology in cases of subjective PT in the absence of physical indicators and a history of predisposing causes is so scant that in these cases, the authors recommend that the clinician discusses a “wait-and-see” approach with the patient. This should involve outlining the risks and costs associated with extensive imaging studies alongside the risks of an undiagnosed underlying pathology.
MRI protocols for basic and comprehensive diagnostic imaging
Two MRI protocols are suggested below depending on the level of clinical suspicion: one, a fast-basic screening test, and the second, a comprehensive protocol with contrast to image the venous system.
Fast and basic MRI screening protocol
- Fluid attenuated inversion recovery (FLAIR) brain;
- Diffusion-weighted imaging (DWI);
- 3D T2 high resolution of the internal auditory canal (IAC);
- Sagittal T1;
- Non-contrast MRA brain.
Comprehensive MRI protocol for PT
In addition to the above imaging protocol:
- Susceptibility-weighted sequence (SWI) which can be helpful in detecting arteriovenous malformations (AVMs);
- Contrast enhanced magnetic resonance venography (MRV);
- Volumetric post contrast T1.
Additional MR sequences such as a dynamic MRA are not routinely performed unless there is a high clinical suspicion for an AVM or if it is suggested by prior imaging.
Radiological findings
Examples of typical radiological findings for the pathologies highlighted in this report are described below.
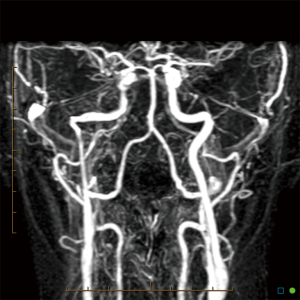
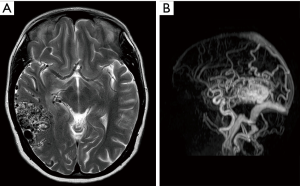
An aberrant ICA may be seen via MRA as a lateralisation of part of the ICA and/or an ICA with a narrowed or “pinched” appearance (Figure 1). DAVF can also be detected via MRA, as in Figure 2, which demonstrates an arterialised left transverse sinus. AVM will be seen in T2 weighted MRI (Figure 3A) and dynamic MRA (Figure 3B) as an asymmetrical vascular tangle.

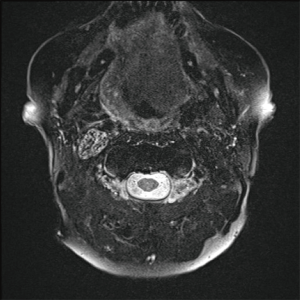
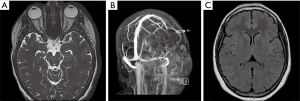
Fat saturated T2-weighted MRI will present a glomus tumour as a bright mass, the mass often causing displacement in proximal vasculature (Figure 4). T2-weighted MRI can also be used in suspected BIH, wherein imaging will demonstrate pathological optic nerves and flattened optic discs (Figure 5A). In the latter case, MRI can be followed-up with MRV, wherein a BIH diagnosis will be supported by the presence of asymmetrical narrowing of the transverse sinus (Figure 5B); and FLAIR imaging, wherein BIH patients will exhibit a narrowing of the lateral ventricles (Figure 5C). Figure S2 provides an age- and sex-matched non-PT patient MRI for comparison.
Finally, abnormal condylar or emissary veins can be visualised through MRV (Figure 6).

Discussion
This review has established that there are drastically conflicting approaches to the diagnosis of PT. This controversy has been driven both by changes in the technological capability of different imaging modalities, and by the complicated nature of PT as a possible symptom for a wide variety of pathologies, ranging from the benign to the life threatening.
We have established that any imaging study must be preceded by a thorough physical examination, including otoscopy, auscultation, blood pressure and select blood tests, audiometry and the use of jugular vein compression or the Valsalva manoeuvre to determine the arterial or venous nature of the symptom. Clinicians should also make efforts to take a comprehensive medical history, with attention to risk factors such as past head trauma or cardiovascular issues; comorbidities presenting with the tinnitus, like vertigo or headaches; and any acute or gradual changes in the symptom.
We have also assessed the utility of MRI, CT and angiography in the diagnosis of PT. We found that approaches to radiography in PT cases should always be directed by initial clinical findings. We have determined that in most cases of PT, modern MRI is the best choice for primary imaging modality due to its superior sensitivity to most PT-causing pathologies and the lower associated morbidity compared to CT or traditional angiography. We have also identified medical scenarios in which the latter two approaches may be useful. We have presented an argument in favour of foregoing imaging in PT cases with no objective symptoms and where no risk factor can be identified in the medical history. Finally, we have offered recommendations for both basic and comprehensive MRI protocols for use in PT diagnosis.
This review provides an up-to-date reference guide for the evaluation of PT, including recommendations for each step of the diagnostic process. It should also stimulate discussion as to the importance of the initial exchange of information between the patient and the clinician in cases where multiple aetiologies may be reasonably suspected for a given symptom.
As with all medical reviews, this study is limited in the breadth of content and selection of studies for review. We focused entirely on cases of vascular PT with little mention of non-vascular PT, and with no attention paid to the equally complex matter of non-PT. We also excluded studies from the original database search that were not written in English, and several case studies that mentioned PT as a symptom but where it was not the presenting symptom or where the nature of the PT was insufficiently discussed. These choices may have skewed the epidemiological statistics for underlying pathologies and may have eliminated discussion of any alternative approaches to diagnosis common in non-English speaking countries.
It should also be noted that not all case studies or reviews in our selection detailed intervention and outcomes for diagnosed pathologies. Several studies in our selection observe that there is a relatively high likelihood of a type I error in PT cases, where a possible cause for the symptom is demonstrated in a scan, but where resolution of the condition does not resolve the PT. The implication is that the suspected cause was not ultimately responsible for the symptom, a possible limitation of our recommendations. It should also be noted that approaching any diagnosis with a heavy dependence on patient’s self-reported medical history is weakened by reliance on the patient’s accurate and comprehensive recall. In PT cases, this may mean a failure to remember a head injury, or presenting an inaccurate timeframe of symptom development, items that may be crucial in the investigation of a dangerous pathology. Nevertheless, we conclude that diagnosis of PT should be driven by comprehensive clinical data and dominated by the conservative use of MRI.
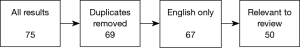
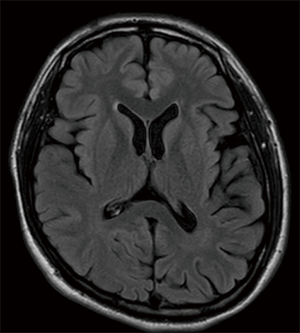
Table S1
| Hyperdynamic circulatory states |
| Thyrotoxicosis |
| Anaemia |
| Fever |
| Aortic valve incompetence |
| Widespread Paget’s disease |
Table S2
| Common MRI findings in BIH |
| Partial empty sella |
| Dural venous sinus stenosis |
| Usually tapered stenosis of the lateral segment of the transverse sinuses |
| Slit-like ventricles |
| Prominent optic nerve sheath |
| Flat optic disc |
| Vertical tortuosity of the optic nerves |
| Increased fat thickness in the neck |
MRI, magnetic resonance imaging; BIH, benign intracranial hypertension.
Acknowledgments
The authors wish to thank Prof. David Ryugo for his invaluable input during the revision process.
Funding: The authors acknowledge funding support from the National Health and Medical Research Council (#1080652), the Oticon Foundation (#15-1814), and a generous donation from Alan and Lynne Rydge, 2018.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.09.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Madani G, Connor SE. Imaging in pulsatile tinnitus. Clin Radiol 2009;64:319-28. [Crossref] [PubMed]
- Levine SB, Snow JB Jr. Pulsatile tinnitus. Laryngoscope 1987;97:401-6. [Crossref] [PubMed]
- Weissman JL, Hirsch BE. Imaging of tinnitus: a review. Radiology 2000;216:342-9. [Crossref] [PubMed]
- Sismanis A. Pulsatile tinnitus: contemporary assessment and management. Curr Opin Otolaryngol Head Neck Surg 2011;19:348-57. [Crossref] [PubMed]
- Herraiz C, Aparicio JM. Diagnostic clues in pulsatile tinnitus (somatosounds). Acta Otorrinolaringol Esp 2007;58:426-33. [Crossref] [PubMed]
- Sonmez G, Basekim CC, Ozturk E, et al. Imaging of pulsatile tinnitus: a review of 74 patients. Clin Imaging 2007;31:102-8. [Crossref] [PubMed]
- Willinsky RA. Tinnitus: imaging algorithms. Can Assoc Radiol J 1992;43:93-9. [PubMed]
- Levine RA. Tinnitus: diagnostic approach leading to treatment. Semin Neurol 2013;33:256-69. [Crossref] [PubMed]
- Hofmann E, Behr R, Neumann-Haefelin T, et al. Pulsatile tinnitus: imaging and differential diagnosis. Dtsch Arztebl Int 2013;110:451-8. [PubMed]
- Remley KB, Harnsberger HR, Jacobs JM, et al. The radiologic evaluation of pulsatile tinnitus and the vascular tympanic membrane. Semin Ultrasound CT MR 1989;10:236-50. [PubMed]
- Shweel M, Hamdy B. Diagnostic utility of magnetic resonance imaging and magnetic resonance angiography in the radiological evaluation of pulsatile tinnitus. Am J Otolaryngol 2013;34:710-7. [Crossref] [PubMed]
- Mundada P, Singh A, Lingam RK. CT arteriography and venography in the evaluation of Pulsatile tinnitus with normal otoscopic examination. Laryngoscope 2015;125:979-84. [Crossref] [PubMed]
- Endo K, Maruyama Y, Tsukatani T, et al. Aberrant internal carotid artery as a cause of objective pulsatile tinnitus. Auris Nasus Larynx 2006;33:447-50. [Crossref] [PubMed]
- Sasindran V, Joseph A, Abraham SS, et al. High-riding jugular bulb: A rare entity. Indian J Otol 2014;20:129-31. [Crossref]
- Baomin L, Yongbing S, Xiangyu C. Angioplasty and stenting for intractable pulsatile tinnitus caused by dural venous sinus stenosis: a case series report. Otol Neurotol 2014;35:366-70. [Crossref] [PubMed]
- Pons Y, Verillaud B, Ukkola-Pons E, et al. Pulsatile tinnitus and venous cerebral thrombosis: report of a case and literature review. Rev Laryngol Otol Rhinol (Bord) 2012;133:163-4. [PubMed]
- Kreuzer PM, Landgrebe M, Vielsmeier V, et al. Trauma-associated tinnitus. J Head Trauma Rehabil 2014;29:432-42. [Crossref] [PubMed]
- Houdart E, Chapot R, Merland JJ. Aneurysm of a dural sigmoid sinus: a novel vascular cause of pulsatile tinnitus. Ann Neurol 2000;48:669-71. [Crossref] [PubMed]
- Friedmann DR, Le BT, Pramanik BK, et al. Clinical spectrum of patients with erosion of the inner ear by jugular bulb abnormalities. Laryngoscope 2010;120:365-72. [PubMed]
- Otto KJ, Hudgins PA, Abdelkafy W, et al. Sigmoid sinus diverticulum: a new surgical approach to the correction of pulsatile tinnitus. Otol Neurotol 2007;28:48-53. [Crossref] [PubMed]
- Dawson J, Morland R, Brooks R. A picture of overweight and obesity in Australia 2017. Canberra, Australia: Australian Institute of Health and Welfare, 2017.
- Liu Z, Dong C, Wang X, et al. Association between idiopathic intracranial hypertension and sigmoid sinus dehiscence/diverticulum with pulsatile tinnitus: a retrospective imaging study. Neuroradiology 2015;57:747-53. [Crossref] [PubMed]
- Panda A, Arora A, Jana M. Persistent primitive trigeminal artery: an unusual cause of vascular tinnitus. Case Rep Otolaryngol 2013;2013:275820. [Crossref] [PubMed]
- Xue J, Li T, Sun X, et al. Focal defect of mastoid bone shell in the region of the transverse-sigmoid junction: a new cause of pulsatile tinnitus. J Laryngol Otol 2012;126:409-13. [Crossref] [PubMed]
- Manski TJ, Heffner DK, Glenn GM, et al. Endolymphatic Sac Tumors: A Source of Morbid Hearing Loss in von Hippel-Lindau Disease. JAMA 1997;277:1461-6. [Crossref] [PubMed]
- Kim DK, Shin YS, Lee JH, et al. Pulsatile tinnitus as the sole manifestation of an internal carotid artery aneurysm successfully treated by coil embolization. Clin Exp Otorhinolaryngol 2012;5:170-2. [Crossref] [PubMed]
- Lee SH, Kim SS, Sung KY, et al. Pulsatile tinnitus caused by a dilated mastoid emissary vein. J Korean Med Sci 2013;28:628-30. [Crossref] [PubMed]
- Pak MW, Kew J, Andrew van Hasselt C. Lateralized carotid artery: an unusual cause of pulsatile tinnitus. Ear Nose Throat J 2001;80:148-9. [PubMed]
- Pelkonen O, Tikkakoski T, Luotonen J, et al. Pulsatile tinnitus as a symptom of cervicocephalic arterial dissection. J Laryngol Otol 2004;118:193-8. [Crossref] [PubMed]
- Marion MS, Cevette MJ. Tinnitus. Mayo Clin Proc 1991;66:614-20. [Crossref] [PubMed]
- Tuz M, Dogru H, Yesildag A. Subjective pulsatile tinnitus associated with extensive pneumatization of temporal bone. Auris Nasus Larynx 2003;30:183-5. [Crossref] [PubMed]
- Sismanis A, Stamm MA, Sobel M. Objective tinnitus in patients with atherosclerotic carotid artery disease. Am J Otol 1994;15:404-7. [PubMed]
- Sila CA, Furlan AJ, Little JR. Pulsatile tinnitus. Stroke 1987;18:252-6. [Crossref] [PubMed]
- Bitoh S, Sakaki S. Spontaneous cure of dural arteriovenous malformation in the posterior fossa. Surg Neurol 1979;12:111-4. [PubMed]
- Chang TP, Wu YC, Hsu YC. Vestibular paroxysmia associated with paroxysmal pulsatile tinnitus: a case report and review of the literature. Acta Neurol Taiwan 2013;22:72-5. [PubMed]
- Mehanna R, Shaltoni H, Morsi H, et al. Endovascular treatment of sigmoid sinus aneurysm presenting as devastating pulsatile tinnitus. A case report and review of literature. Interv Neuroradiol 2010;16:451-4. [Crossref] [PubMed]
- Dietz RR, Davis WL, Harnsberger HR, et al. MR imaging and MR angiography in the evaluation of pulsatile tinnitus. AJNR Am J Neuroradiol 1994;15:879-89. [PubMed]
- Mehall CJ, Wilner HI, LaRouere MJ. Pulsatile tinnitus associated with a laterally placed sigmoid sinus. AJNR Am J Neuroradiol 1995;16:905-7. [PubMed]
- Piepgras DG, McGrail KM, Tazelaar HD. Intracranial dissection of the distal middle cerebral artery as an uncommon cause of distal cerebral artery aneurysm. Case report. J Neurosurg 1994;80:909-13. [Crossref] [PubMed]
- Pirodda A, Sorrenti G, Marliani AF, et al. Arterial anomalies of the middle ear associated with stapes ankylosis. J Laryngol Otol 1994;108:237-9. [Crossref] [PubMed]
- Rosenbloom JS, Storper IS, Aviv JE, et al. Giant cell tumors of the jugular foramen. Am J Otolaryngol 1999;20:176-9. [Crossref] [PubMed]
- Santa Maria PL. Sigmoid sinus dehiscence resurfacing as treatment for pulsatile tinnitus. J Laryngol Otol 2013;127:S57-9. [Crossref] [PubMed]
- Xenellis J, Nikolopoulos TP, Felekis D, et al. Pulsatile tinnitus: a review of the literature and an unusual case of iatrogenic pneumocephalus causing pulsatile tinnitus. Otol Neurotol 2005;26:1149-51. [Crossref] [PubMed]
- Adelman JU. Headaches and papilledema secondary to dural arteriovenous malformation. Headache 1998;38:621-3. [Crossref] [PubMed]
- Reece PH, Higgins N, Hardy DG, et al. An aneurysm of the petrous internal carotid artery. J Laryngol Otol 1999;113:55-7. [Crossref] [PubMed]
- Gruber B, Hemmati M. Fibromuscular dysplasia of the vertebral artery: an unusual cause of pulsatile tinnitus. Otolaryngol Head Neck Surg 1991;105:113-4. [Crossref] [PubMed]
- Biller J, Sacco RL, Albuquerque FC, et al. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2014;45:3155-74. [Crossref] [PubMed]
- Crummer RW, Hassan GA. Diagnostic approach to tinnitus. Am Fam Physician 2004;69:120-6. [PubMed]
- De Ridder D, De Ridder L, Nowe V, et al. Pulsatile tinnitus and the intrameatal vascular loop: why do we not hear our carotids? Neurosurgery 2005;57:1213-7; discussion 1213-7. [Crossref] [PubMed]
- Grewal AK, Kim HY, Comstock RH 3rd, et al. Clinical presentation and imaging findings in patients with pulsatile tinnitus and sigmoid sinus diverticulum/dehiscence. Otol Neurotol 2014;35:16-21. [Crossref] [PubMed]
- Shin EJ, Lalwani AK, Dowd CF. Role of angiography in the evaluation of patients with pulsatile tinnitus. Laryngoscope 2000;110:1916-20. [Crossref] [PubMed]
- Review of funding for diagnostic imaging services: final report. Canberra, Australia: Medical Benefits Reviews Task Group, Diagnostic Imaging Review Team, Department of Health and Ageing, 2011:1-26. Available online: https://www.aph.gov.au/DocumentStore.ashx?id=3af9956d-9e28-4855-a75f-c7e4f4ac2419&subId=252414
- Radcliffe J, Dibley H, Wrest J, et al. Availability and accessibility of diagnostic imaging equipment around Australia. In: Secretariat SCAC, editor. Canberra, Australia: Senate Printing Unit; 2018. p. 10, 27, 38, 9, 41. Available online: https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/Diagnosticimaging/Report
- Daneshi A, Hadizadeh H, Mahmoudian S, et al. Pulsatile tinnitus and carotid artery atherosclerosis. Int Tinnitus J 2004;10:161-4. [PubMed]
- HealthDirect. MRI Scan. Australian Government, Canberra, Australia. 2017. Accessed 02/08/2018. Available online: https://www.healthdirect.gov.au/magnetic-resonance-imaging-mri
- HealthDirect. CT Scan. Canberra, Australia. 2017. Accessed 02/08/2018. Available online: https://www.healthdirect.gov.au/ct-scan
- HealthDirect. Angiogram. Australian Government, Canberra, Australia. 2017. Accessed 02/08/2018. Available online: https://www.healthdirect.gov.au/angiogram
- Medicare-Australia. MBS Online. In: Medicare Benefits Schedule Australian Government Department of Health, Canberra, Australia. 2018. Accessed 02/08/2018. Available online: http://www9.health.gov.au/mbs/search.cfm
- Wang GP, Zeng R, Ma XB, et al. Surgical treatment of pulsatile tinnitus caused by the sigmoid sinus diverticulum: a preliminary study. Medicine (Baltimore) 2015;94:e882. [Crossref] [PubMed]
- Cho IK, Jung JY, Yoo DS, et al. 3-Dimensional reconstruction of the venous system in patients suffering from pulsatile tinnitus. Acta Otolaryngol 2012;132:285-9. [Crossref] [PubMed]
Cite this article as: Grierson KE, Bou-Haidar P, Dumper J, Fagan PA. The assessment of pulsatile tinnitus—a systematic review of underlying pathologies and modern diagnostic approaches. Aust J Otolaryngol 2018;1:27.


