
The analgesic efficacy of cyclooxygenase-2 selective inhibitors in paediatric adenotonsillectomy: a systematic review
Introduction
Adenotonsillectomy remains one of the most common paediatric surgeries in Australia, with more than 38,000 cases performed annually (1). The rate of paediatric adenotonsillectomy will likely continue to grow as the indications for surgical management shift from recurrent tonsillitis towards the common treatment of sleep-disordered breathing and obstructive sleep apnoea (OSA) (2).
Despite improvements over time in surgical technique and hospital admission and complication rates, postoperative analgesia still remains a challenging aspect in the management of these patients. Due to narrow paediatric tolerance levels there are many barriers to effective postoperative analgesia. Current therapies such as opioids or traditional non-steroidal anti-inflammatory (NSAIDS) may be limited by undesirable side effects such as sedation, respiratory depression, nausea and vomiting or interference with coagulation (3-5). Complicating matters further, regular and strong pain relief is usually required for over one week coinciding with maximum fibrin clot accumulation and subsequent breakdown at this time (6). Pain can be severe and inevitably effects a young patient’s oral intake, overall health status and ability to assume normal activities of daily life (7,8).
For these reasons, there has been interest in the use of newer selective cyclooxygenase-2 (COX-2) inhibitors for post-operative analgesia in paediatric adenotonsillectomy. Drugs of this class reduce the production of inflammatory prostaglandins via their selective blockade of COX-2, whilst sparing prostaglandins that maintain platelet aggregation and gastric mucosal integrity via their low affinity for COX-1 (9). One significant concern regarding the selective COX-2 inhibitors is the risk of atherothrombosis that has been demonstrated in the adult population. They have faced scrutiny by therapeutic regulatory boards in certain countries resulting in some agents being pulled from existing markets (10). Even though the risk of atherothrombosis in children is presumably low, it is unlikely a study would ever be conducted to prove this. In Australia the Therapeutic Goods Association (TGA) has not approved the use of celecoxib in any paediatric populations (11) despite other international regulatory boards clearing it for use in specific paediatric cases (12). Unsurprisingly, there remains little in terms of data on efficacy of COX-2 inhibitors in paediatric populations with practitioners instead opting for their “off-label” use. To complicate matters further, there are no standardised paediatric dosing regimens, including preferred route, dose strength and duration/frequency of treatment.
This systematic review aims to assess the relevant current literature regarding the efficacy of COX-2 inhibitors in the treatment of post-operative pain following paediatric adenotonsillectomy in an attempt to answer some of these questions.
Methods
Eligibility criteria
Inclusion and exclusion criteria were predefined. Inclusion criteria included English language, human studies with participants younger than 18 years of age that analysed the efficacy of selective COX-2 inhibitors in postoperative analgesia following adenotonsillectomy. Case reports or series, letters to the editor and abstracts were included if they held adequate data. Non-English, review articles and any in vitro or animal studies were excluded from this review.
Types of interventions
Selective COX-2 inhibitors/antagonists may be administered either orally or parentally. These include celecoxib (Celebrex, Pfizer), rofecoxib (Vioxx, Merck), valdecoxib (Bextra, Pfizer) and parecoxib (Xaptek, Pfizer).
Types of outcome measures
Primary
Efficacy of pain relief with COX-2 inhibitor/antagonist in paediatric patients following adenotonsillectomy as defined by:
- Subjective measurements
- Pain scores (visual analogue scales; standardised pain scores/scales/journals; parental checklists);
- Satisfaction (standardised questionnaire or otherwise stated).
Secondary
- Surrogate outcomes;
- Use of adjunct pain medication;
- Time to full recovery/activity;
- Length of hospital admission (recovery/ward discharge).
- Adverse events associated with treatment.
- Haemorrhage;
- Side effects (post-operative nausea and vomiting, drug reactions);
- Readmission.
Search strategy
A systematic search was performed by using the PubMed, MEDLINE, and EMBASE databases. The PubMed database was searched from inception until December 1, 2017; EMBASE was searched from 1974 until December 1, 2017, and MEDLINE was searched from 1946 to December 1, 2017 by using Ovid SP. Bibliographies of studies selected for full-text analysis were reviewed for any additional missing studies. An electronic search strategy was designed to identify all studies concerned with the efficacy of COX-2 inhibitors/antagonists in postoperative pain relief following paediatric adenotonsillectomy (see Appendix 1: search strategy).
Data collection and analysis
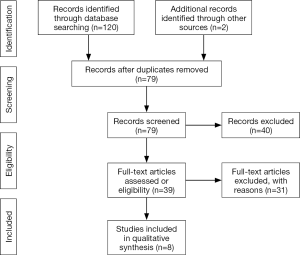
Two unblinded reviewers reviewed the titles and abstracts, read full-text articles and evaluated them against the inclusion criteria. Studies that met the inclusion criteria had the relevant data extracted using a standardised data form. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for study selection is shown in Figure S1.
The review authors conducted the data extraction and assessed the quality of the method used in each included trial. Considered factors were:
- Number of participants;
- Age of participants;
- Sociodemographic data;
- Characteristics of trial (e.g., method of randomisation, blinding, the use of intention-to-treat analysis);
- Inclusion and exclusion criteria;
- Risk of bias;
- Diagnostic criteria;
- Treatment: preoperative, intraoperative and postoperative;
- Adjunct treatment: preoperative, intraoperative and postoperative;
- Duration and type of treatment;
- Outcome measures;
- Follow-up period;
- Adverse effects.
Risk of bias
Risk of bias in randomised controlled trials (RCTs) was assessed at a study level using the Cochrane Risk of Bias Assessment Tool. For cohort studies, risk of bias was assessed in accordance with the Newcastle-Ottawa Scale (13).
Analysis
A statistician was consulted regarding the applicability of a meta-analysis to the current data. Given the heterogeneity of treatment interventions, outcome measurements and duration of follow up, qualitative review was deemed to be a more appropriate form of data analysis and a meta-analysis would be foregone.
Results
Search
A total of 122 references were identified by the search. First level screening removed 83 studies (e.g., removal of duplicates, non-English, clearly irrelevant scope) leaving 39 references for full text consideration. A further 31 publications were excluded because they did not meet the inclusion criteria. Eight articles were chosen for final review (14-21) (six RCTs, a randomised trial lacking control and a prospective comparative study) (see Table 1). Of these included papers, 5 analysed oral rofecoxib (17-21), 2 intravenous parecoxib (14,15) and 1 oral celecoxib (16) for postoperative analgesia in adenotonsillectomy.
Table 1
| Author, year | Type of study | Cohort | Route | COX-2 agent | Adjunct pain medication | Comparison |
|---|---|---|---|---|---|---|
| Tan et al., 2016 | RCT | 59 | Intravenous | Parecoxib 0.25/0.5/1 mg/kg | Tonsillar block#, tramadol*; acetaminophen^, tramadol^; morphine* | Parecoxib |
| Li et al., 2016 | RCT | 60 | Intravenous | Parecoxib 1 mg/kg perioperatively | Morphine#*; paracetamol^; tramadol^ | Placebo |
| Murto et al., 2015 | RCT | 195 | Oral | Celecoxib 6 mg/kg preoperatively | Acetaminophen^; morphine*^ | Placebo |
| Celecoxib 3 mg/kg BD for 5 doses | ||||||
| Vallée et al., 2007 | Prospective comparative study | 80 | Oral | Rofecoxib 1 mg/kg 5/7 | Morphine* | Acetaminophen |
| Bean-Lijewski et al., 2007 | RCT | 40 | Oral | Rofecoxib 1 mg/kg 3/7 | Fentanyl*; hydromorphone/acetaminophen^; acetaminophen^ | Hydromorphone/acetaminophen |
| Sheeran et al., 2004 | RCT | 45 | Oral | Rofecoxib 1 mg/kg preoperatively | Morphine* | Placebo |
| Joshi et al., 2003 | RCT | 66 | Oral | Rofecoxib 1 mg/kg preoperatively | Fentanyl*; acetaminophen/codeine* | Placebo |
| Pickering et al., 2002 | RCT | 98 | Oral | Rofecoxib 0.625 mg/kg preoperatively | Paracetamol*; ibuprofen*; codeine* | Placebo, ibuprofen |
#, intraoperative; *, inpatient; ^, outpatient. COX-2, cyclooxygenase-2; RCT, randomised controlled trial; 3/7, 3 days duration; 5/7, 5 days duration.
Study objectives
Six papers analysed pain as a primary outcome (15-18,20,21). Rofecoxib was compared to placebo (19-21), ibuprofen (21), acetaminophen (17) and a combination of acetaminophen/hydromorphone (18) whilst celecoxib was analysed against a placebo (16). Parecoxib was analysed at incremental strengths (14) and against a placebo (15).
The two remaining papers primarily analysed the pharmacokinetic profile of intravenous parecoxib (14) and the rate of intraoperative bleeding with oral rofecoxib (19). Pain scores were a common secondary outcome.
Other secondary objectives included surrogate measures of pain (adjunct pain relief requirements, time to discharge and full recovery), side effects (haemorrhage, nausea and vomiting and general) and levels of satisfaction with treatment (questionnaire or otherwise stated).
Interventions
Rofecoxib was administered orally either as a single preoperative dose or as a postoperative course ranging from three to five days. Dose strength was either 0.625 or 1 mg/kg daily. Follow-up ranged from 24 hours to 1-week post operation (17-21).
Parecoxib was administered intravenously as a single preoperative dose at 0.25, 0.50 or 0.1 mg/kg. Follow-up was at 24 hours (14,15).
Celecoxib was administered at two strengths; 6 mg/kg as a single oral dose preoperatively, and 3 mg/kg for 5 oral doses postoperatively. This was on a twice daily dosing schedule. Follow-up occurred at days 1 and 2, at 1 and 2 weeks and at 5 months post adenotonsillectomy (16).
Adjunct pain relief varied widely in the preoperative, intraoperative and postoperative setting (see Table 1).
Outcomes
Efficacy of pain relief
Pain relief was analysed via several scoring systems and surrogate measures including use of adjunct pain relief, time to discharge and time to full recovery (see Table 2).
Table 2
| Study | Setting | Pain score | Pain findings | Time to recovery | Additional pain relief | Satisfaction |
|---|---|---|---|---|---|---|
| Tan et al., 2016 | Inpatient | Faces Pain Scale | Nil difference in pain scores | Not recorded | Nil difference in use of tramadol, morphine or fentanyl | Not recorded |
| Li et al., 2016 | Inpatient | CHEOPS | Significantly less pain with parecoxib compared to control (P=0.001) | Not recorded | Less daily (P=0.024) and cumulative total dosages (P=0.003) of morphine with parecoxib compared to control | Not recorded |
| Significantly shorter time to rescue medication in placebo group (P=0.001) | ||||||
| Murto et al., 2015 | Inpatient; outpatient | VAS; parental checklist (<5 years) | Significantly less “worst pain” scores over first 24 hours with celecoxib (P=0.01) | Nil difference in time to full recovery | Nil difference in cumulative acetaminophen or morphine use | Nil difference between groups |
| Nil difference in pain scores at any other day analyzed (P=0.10) | ||||||
| Vallée et al., 2007 | Outpatient | VAS | Significantly lower pain scores at day 0 (P<0.0001), 1 (P=0.0010) and 3 (P=0.0027) with rofecoxib compared to acetaminophen | Faster time to recovery in patients treated with rofecoxib compared to acetaminophen (P<0.05) | Nil difference in daily dosing of morphine | Not recorded |
| Bean-Lijewski et al., 2007 | Inpatient; outpatient | ACCS | Significantly lower active pain scores over first 3 days with rofecoxib (P<0.05) | Not recorded | Not recorded | Not recorded |
| Nil difference in passive pain scores | ||||||
| Sheeran et al., 2004 | Inpatient | CHEOPS | Nil difference in pain scores | Not recorded | Nil difference in morphine consumption | Greater satisfaction with rofecoxib compared to placebo |
| Joshi et al., 2003 | Inpatient | Wong and Baker | Significantly lower early pain scores at 2 and 24 hours with rofecoxib (P<0.05; P<0.0006) | Not recorded | Nil difference in codeine and morphine between groups | Not recorded |
| Pickering et al., 2002 | Inpatient | Oucher pain scale | Nil difference in pain scores | Not recorded | Ibuprofen required significantly less pain relief first 2 hours postoperatively (P=0.03) | Not recorded |
| Nil difference in cumulative use |
CHEOPS, Children’s Hospital of Eastern Ontario Pain Scale; VAS, Visual Analogue Scale; ACCS, Analogue Continuous Chromatic Scale.
Five different scoring systems were used when analysing the efficacy of rofecoxib (17-21); three papers showed significantly lower pain scores with rofecoxib compared to placebo, acetaminophen and acetaminophen/hydromorphone respectively for the designated study period (17,18,20) (see Table 1). The remaining two papers did not show any significant difference in pain scores compared to placebo and ibuprofen (19,21). No papers showed any significant difference in the use of adjunct pain relief with rofecoxib compared to its control (17-21).
Parecoxib was shown to significantly decrease pain scores and cumulative acetaminophen and morphine dosaging post-adenotonsillectomy compared to placebo (15). Stronger dosaging did not correlate with either of these findings in a separate paper (14).
Celecoxib reduced early postoperative pain scores and analgesic consumption. This was not significant for the duration of the study period (16).
Only two papers analysed time to full recovery, finding either no difference with celecoxib compared to placebo (16) or improved with rofecoxib compared to acetaminophen (17).
Adverse effects
Seven papers analysed rates of haemorrhage (14-17,19-21) (see Table 3). Six studies showed no difference in haemorrhage (14,16,17,19-21) whilst Li et al. recorded no cases of post-operative bleeding (15).
Table 3
| Study | Adjunct therapy | Haemorrhage | Nausea and vomiting | Other |
|---|---|---|---|---|
| Tan et al., 2016 | Dexamethasone; ondansetron | Nil difference in bleeding rates | Nil difference | No difference in other side effects |
| Li et al., 2016 | Dexamethasone; ondansetron | No postoperative bleeding | Significantly more nausea and vomiting with placebo (P=0.037) | Sedation scores less in parecoxib group in PACU (P=0.032) |
| Murto et al., 2015 | Dexamethasone; ondansetron | Nil difference in bleeding rates | Nil difference | No difference in other side effects |
| Vallée et al., 2007 | Nil recorded | Nil difference in bleeding rates | Not recorded | No side effects reported |
| Bean-Lejewski et al., 2007 | Dexamethasone; ondansetron | Not recorded | Not recorded | No difference in other side effects |
| Sheeran et al., 2004 | Dexamethasone; ondansetron | Nil difference in bleeding rates | Nil difference | No difference in other side effects |
| Joshi et al., 2003 | Dexamethasone; dolasetron | Nil difference in bleeding rates | Significantly more nausea and vomiting in control group post discharge (P<0.05) | No difference in other side effects |
| Pickering et al., 2002 | Not given preoperatively | Nil difference in bleeding rates (P=0.30) | Nil difference (P=0.71) | Not recorded |
PACU, post-anesthesia care unit.
Six studies analysed post-operative nausea and vomiting with the majority showing no difference compared to their respective controls (14-16,19-21) (see Table 3). Li et al. showed a significant reduction in immediate in hospital post-operative nausea and vomiting and sedation levels with parecoxib compared to control (15). Joshi et al. found rofecoxib had reduced outpatient nausea and vomiting rates compared to placebo (20).
Levels of satisfaction
Levels of satisfaction were recorded in two papers (16,19). Murto et al. showed that celecoxib did not improve patient satisfaction compared to placebo, but patients did have an improved emotional and physical recovery according to quality of life questionnaires (16). Sheeran et al. stated a higher level of parent satisfaction with rofecoxib compared to control without referencing a tool of measurement (19).
Risk of bias
Comprehensive reporting of study methodologies was inconsistent amongst the included studies (see Table 4). All papers provided adequate evidence of sequence generation. Only two studies stated adequate measures to conceal allocation (14,15). All but one study (19) blinded patients effectively and were free of selective reporting. Two studies did not adequately account for incomplete data dropouts (18,19). All studies, due to the nature of pain scoring, suffer from bias inherent in subjective measurements through scoring systems and second-degree reporting (parents).
Table 4
| Study | Adequate sequence generation | Allocation concealment | Blinding | Incomplete outcome data addressed | Free of selective outcome reporting | Free of other sources of bias |
|---|---|---|---|---|---|---|
| Tan et al., 2016 | Yes | Yes | Yes | Yes | Yes | No |
| Li et al., 2016 | Yes | Yes | Yes | Yes | Yes | No |
| Murto et al., 2015 | Yes | Unclear | Yes | Yes | Yes | No |
| Bean-Lijewski et al., 2007 | Yes | Unclear | Yes | Unclear | Yes | No |
| Sheeran et al., 2004 | Yes | Unclear | Unclear | Unclear | Unclear | No |
| Joshi et al., 2003 | Yes | Unclear | Yes | Yes | Yes | No |
| Pickering et al., 2002 | Yes | Unclear | Yes | Yes | Yes | No |
RCT, randomised controlled trial.
One non-RCT (comparative cohort) was of good quality in terms of selection and comparability (17) (see Table 5). However, there was no evidence of adequate blinding of assessors.
Table 5
| Study | Representativeness of the exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Comparability of cohorts | Assessment of outcome | Follow-up 1 week | Adequacy of follow-up |
|---|---|---|---|---|---|---|---|---|
| Vallée et al., 2007 | * | * | * | * | * | # | * | * |
*, noted in study; #, not noted in study.
Two RCT’s (20,21) and the one included cohort study (17) did not declare conflict of interests or funding sources. All the remaining RCT’s addressed conflict of interest, funding source and/or sponsorship (14-16,18,19).
Discussion
Despite the available data suggesting that COX-2 specific inhibitors may be safe and effective post analgesic agents, the paucity and heterogeneity of the data limit any true findings.
The efficacy of rofecoxib in postoperative adenotonsillectomy analgesia remains somewhat contradictory. The majority of studies analysing rofecoxib showed a significant reduction in pain scores including both papers in an outpatient setting (17,18,20). Rofecoxib has a delayed peak action (2–3 hours) and half-life (17 hours), meaning that the greatest benefit may not be experienced until after discharge (22). This may explain the equivocal findings of earlier single dose studies that only analysed in patient analgesia (19,21).
Despite rofecoxib being the most well studied of the COX-2 inhibitors, there still remains little to no data on dosing. The landmark paper from Pickering et al. based the rofecoxib dose off a ratio equivalent to that used for paediatric ibuprofen (21). However, children have faster clearance and volume distribution of rofecoxib and celecoxib compared to adults, meaning that this proposed dosing in earlier studies may be too small and underestimate the true analgesic response (15,23). Daily dosing for approximately 1-week post operatively appeared to be a safe and effective method of analgesia according to several more recent papers (17,18).
Interestingly no studies showed an opioid sparing effect with rofecoxib (17-21). A reason cited by several authors was parental concern in over medicating (24). This meant parents were prone to under or over utilise opioids depending on the regularity in which they were receiving their primary medication, with parents less likely to use opioids when on regular acetaminophen. However poor study design and short follow up periods deem these findings far from conclusive (17,20-21). Even with all this in mind, rofecoxib still remains off market after being pulled by its manufacturer Merck in 2004 due to its increased risk of atherothrombotic disease in adults.
The two studies that analysed the efficacy of intravenous parecoxib in immediate (24 hours) postoperative analgesia had conflicting results. Li et al. showed a significant reduction in pain scores, cumulative analgesia (acetaminophen and morphine) and significantly less side effects (sedation, nausea and vomiting) with single dose parecoxib compared to placebo (15). The authors stated that parecoxib had an opioid sparing effect with minimal side effects that made it an attractive option in perioperative analgesia. More recently, Tan et al. investigated the pharmacokinetic profile of incremental doses of intravenous parecoxib and were unable to demonstrate a significant change in pain scores or use of adjunct pain medications relating to strength (14). This highlights the short comings in studying immediate post-operative pain relief, with several agents often used intraoperatively by anaesthetist and surgeon that may confound and obviate the need for initial post-operative analgesia. A noteworthy finding was that unlike oral agents celecoxib and rofecoxib, parecoxib had a similar pharmacokinetic profile in children as it did adults (14).
Murto et al. remains the only paper to analyse celecoxib’s use in post-adenotonsillectomy paediatric analgesia, with the primary outcome evaluating pain over the first 24 hours (16). They showed a significant reduction in worst pain scores and use of adjunct medication immediately post operatively. However, there was no difference in pain relief, time to full recovery, use of adjunct medications or levels of satisfaction compared to placebo over the first week of follow-up. The half-life of celecoxib in children is reduced (5 hours compared to eleven hours in adults) and clearance is doubled, meaning the twice daily dosing in adults was less effective in this paediatric cohort (25,26). The authors concluded celecoxib acted well to blunt the initial pain response, but had minimal other post-operative analgesic effect despite being well tolerated. They stated patients may benefit from “more frequent dosing” (16). Celecoxib has been deemed safe for use in certain paediatric populations (11), but is not cleared for postoperative paediatric analgesia by the TGA (12). Off label it is used by several institutions including the Royal Children’s Hospital in Melbourne, Australia.
Despite the controversy surrounding certain COX-2 specific agents, it has been proven that any chronic use of NSAID poses a higher risk of atherothrombosis in adults. Yet this risk seems to be exceptionally small in a paediatric population with no cardiac risk factors, especially considering the small time frames of treatment. It is therefore no surprise that none of the included studies were able to demonstrate significant adverse effects with the use of any of the COX-2 inhibitors. This ranged from serious side effects of atherothrombosis and haemorrhage, through to post-operative nausea and vomiting (which was improved in several studies with COX-2 inhibitors (15,20) (see Table 3).
This review highlights the paucity, heterogeneity and poor study design inherent in many of the included studies. Firstly, postoperative analgesic regimes and follow-up varied widely between studies (see Table 1) making holistic comparison difficult. In addition, perioperative analgesic techniques (e.g., tonsillar blocks and intravenous analgesia administered by anaesthetists) was inconsistently reported. Electrocautery was the predominant method of tonsillar dissection (15,16,18,20), yet all these studies failed to detail the specific type (monopolar versus bipolar) and strength of cautery used. This may have implications for pain and other post-operative complications such as haemorrhage (27). The included studies neglected to analyse cohorts based on other confounding variables including the age of the patients (adolescent versus early childhood) and the indication for surgery (recurrent tonsillitis versus OSA). These considerations should be factored into future research in this realm.
To our knowledge, there is one paper that investigates the efficacy of the only orally available COX-2 inhibitor currently cleared for paediatric use in Australia (celecoxib) (16), clearly indicating a need for more analysis. Several studies were at significant risk of bias, suffering from poor study design and were underpowered (see Tables 4,5). Rofecoxib has the most data, yet it is difficult to compare studies due to unique pain scoring systems (all with inherent subjective flaws), different dosing, alternative adjunct analgesia and follow up periods (see Table 1). Furthermore, rofecoxib has been pulled from the market following scrutiny over potential serious side effects. Parecoxib is only analysed in the perioperative setting limiting its clinical significance when considering pain is often worst several days post adenotonsillectomy.
Conclusions
Current data remains scarce and poor surrounding the efficacy of COX-2 inhibitors in post-operative paediatric adenotonsillectomy analgesia. Several agents have been analysed, yet heterogeneous and weak study design precludes any definitive conclusions from being made. Compounding this, certain agents are not available in several countries, including Australia due to proposed serious side effects seen in adults. Yet adenotonsillectomy remains a common paediatric surgery, and postoperative pain poses a significant challenge in achieving optimal management. With this in mind, larger longitudinal or randomised and prospective trials looking at outpatient pain relief with COX-2 inhibitors will provide clinical relevance and may improve this complex problem.
Appendix 1 Search strategy
Search strategy: MEDLINE (OVID)
- 01. (Tonsillectomy OR Adenoidectomy OR Adenotonsillectomy). af.
- 02. (cyclooxygenase-2 antagonist OR COX-2 antagonist OR cyclooxygenase-2 inhibitor OR COX-2 inhibitor OR celebrex OR celecoxib OR Bextra OR valdecoxib OR Vioxx OR rofecoxib OR xaptek OR parecoxib). af.
- 03. 1 and 2.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2018.05.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Australian Commission on Safety and Quality in Health Care, Australian Atlas of Healthcare Variance: Tonsillectomy Hospital Admissions 17 years and Under [published 10 December 2015, accessed 4 December 2017]. Available online: https://www.safetyandquality.gov.au/wp-content/uploads/2015/11/SAQ201_04_Chapter3_v6_FILM_tagged_merged_3-6.pdf
- McGahan L, Scott A. Tonsillectomy, Adenoidectomy and Adenotonsillectomy for Obstructive Sleep Apnoea: Review of Clinical Evidence and Guidelines. Royal Australian College of Surgeons. [published 26 March, 2015, accessed 4 December 2017]. Available online: https://www2.health.vic.gov.au/Api/downloadmedia/%257B087B15F0-0A1B-476C-A0A4-BAFC6E4EA887%257D+&cd=3&hl=en&ct=clnk&gl=au
- Marret E, Kurdi O, Zufferey P, et al. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology 2005;102:1249-60. [Crossref] [PubMed]
- Mukherjee K, Esuvaranathan V, Streets C, et al. Adenotonsillectomy in children: a comparison of morphine and fentanyl for peri-operative analgesia. Anaesthesia 2001;56:1193-7. [Crossref] [PubMed]
- Lewis S, Nicholson A, Cardwell ME, et al. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst Rev 2003;CD003591. [PubMed]
- Isaacson G. Pediatric tonsillectomy: an evidence-based approach. Otolaryngol Clin North Am 2014;47:673-90. [Crossref] [PubMed]
- Stewart DW, Ragg PG, Sheppard S, et al. The severity and duration of postoperative pain and analgesia requirements in children after tonsillectomy, orchidopexy, or inguinal hernia repair. Paediatr Anaesth 2012;22:136-43. [Crossref] [PubMed]
- Kotiniemi LH, Ryhänen PT, Moilanen IK. Behavioural changes following routine ENT operations in two-to-ten-year-old children. Paediatr Anaesth 1996;6:45-9. [Crossref] [PubMed]
- Ng TT, Diamantaras D, Priestley J, et al. Is celecoxib a useful adjunct in the treatment of post-tonsillectomy pain in the adult population? A randomised, double-blind, placebo-controlled study. J Laryngol Otol 2017;131:S18-28. [Crossref] [PubMed]
- Martínez-González J, Badimon L. Mechanisms underlying the cardiovascular effects of COX-inhibition: benefits and risks. Curr Pharm Des 2007;13:2215-27. [Crossref] [PubMed]
- Therapeutic Goods Administration (TGA). Australian Public Assessment Report for Celecoxib. [Published 23 June 2010, accessed 7 December 2017]. Available online: https://www.tga.gov.au/sites/default/files/auspar-celebrex.pdf
- Sobel RE, Lovell DJ, Brunner HI, et al. Safety of celecoxib and nonselective nonsteroidal anti-inflammatory drugs in juvenile idiopathic arthritis: results of the Phase 4 registry. Pediatr Rheumatol Online J 2014;12:29. [Crossref] [PubMed]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration [published March 2011, accessed 7 December 2017]. Available online: http://handbook-5-1.cochrane.org/
- Tan L, Taylor E, Hannam JA, et al. Pharmacokinetics and analgesic effectiveness of intravenous parecoxib for tonsillectomy ± adenoidectomy. Paediatr Anaesth 2016;26:1126-35. [Crossref] [PubMed]
- Li X, Zhou M, Xia Q, et al. Parecoxib sodium reduces the need for opioids after tonsillectomy in children: a double-blind placebo-controlled randomized clinical trial. Can J Anaesth 2016;63:268-74. [Crossref] [PubMed]
- Murto K, Lamontagne C, McFaul C, et al. Celecoxib pharmacogenetics and pediatric adenotonsillectomy: a double-blinded randomized controlled study. Can J Anaesth 2015;62:785-97. [Crossref] [PubMed]
- Vallée E, Carignan M, Lafrenaye S, et al. Comparative study of acetaminophen-morphine versus rofecoxib-morphine for post-tonsillectomy pain control. J Otolaryngol 2007;36:264-9. [Crossref] [PubMed]
- Bean-Lijewski JD, Kruitbosch SH, Hutchinson L, et al. Post-tonsillectomy pain management in children: can we do better? Otolaryngol Head Neck Surg 2007;137:545-51. [Crossref] [PubMed]
- Sheeran PW, Rose JB, Fazi LM, et al. Rofecoxib administration to paediatric patients undergoing adenotonsillectomy. Paediatr Anaesth 2004;14:579-83. [Crossref] [PubMed]
- Joshi W, Connelly NR, Reuben SS, et al. An evaluation of the safety and efficacy of administering rofecoxib for postoperative pain management. Anesth Analg 2003;97:35-8. [Crossref] [PubMed]
- Pickering AE, Bridge HS, Nolan J, et al. Double-blind, placebo-controlled analgesic study of ibuprofen or rofecoxib in combination with paracetamol for tonsillectomy in children. Br J Anaesth 2002;88:72-7. [Crossref] [PubMed]
- Chang DJ, Fricke JR, Bird SR, et al. Rofecoxib versus codeine/acetaminophen in postoperative dental pain: a double-blind, randomized, placebo- and active comparator-controlled clinical trial. Clin Ther 2001;23:1446-55. [Crossref] [PubMed]
- Litalien C, Jacqz-Aigrain E. Risks and benefits of nonsteroidal anti-inflammatory drugs in children: a comparison with paracetamol. Paediatr Drugs 2001;3:817-58. [Crossref] [PubMed]
- Finley GA, McGrath PJ, Forward SP, et al. Parents' management of children's pain following 'minor' surgery. Pain 1996;64:83-7. [Crossref] [PubMed]
- Stempak D, Gammon J, Klein J, et al. Single-dose and steady-state pharmacokinetics of celecoxib in children. Clin Pharmacol Ther 2002;72:490-7. [Crossref] [PubMed]
- Krishnaswami S, Hutmacher MM, Robbins JL, et al. Dosing celecoxib in pediatric patients with juvenile rheumatoid arthritis. J Clin Pharmacol 2012;52:1134-49. [Crossref] [PubMed]
- Walker P, Gillies D. Post-tonsillectomy hemorrhage rates: are they technique-dependent? Otolaryngol Head Neck Surg 2007;136:S27-31. [Crossref] [PubMed]
Cite this article as: Stokes P, Guirguis M, Page D. The analgesic efficacy of cyclooxygenase-2 selective inhibitors in paediatric adenotonsillectomy: a systematic review. Aust J Otolaryngol 2018;1:16.


